|
fipronil
Insecticide
IRAC 2B; fiprole
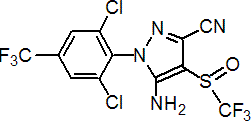
NOMENCLATURE
Common name fipronil (BSI, pa E-ISO)
IUPAC name (?-5-amino-1-(2,6-dichloro-a,a,a-trifluoro-p-tolyl)-4-trifluoromethylsulfinylpyrazole-3-carbonitrile
Chemical Abstracts name 5-amino-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-[(1R,S)-(trifluoromethyl)sulfinyl]-1H-pyrazole-3-carbonitrile
CAS RN [120068-37-3] Development codes MB 46030; RPA-030 (both Rhône-Poulenc)
PHYSICAL CHEMISTRY
Mol. wt. 437.2 M.f. C12H4Cl2F6N4OS Form White solid. M.p. 200-201 ºC; (tech., 195.5-203 °C) V.p. 3.7 ´ 10-4 mPa (25 ºC) KOW logP = 4.0 (shake flask method) Henry 3.7 ´ 10-5 Pa m3 mol-1 (calc.) S.g./density 1.477-1.626 (20 °C) Solubility In water 1.9 (pH 5), 2.4 (pH 9), 1.9 (distilled) (all in mg/l, 20 °C). In acetone 545.9, dichloromethane 22.3, hexane 0.028, toluene 3.0 (all in g/l, 20 °C). Stability Stable in water at pH 5 and 7; slowly hydrolysed at pH 9 (DT50 c. 28 d). Stable to heat. Slowly degrades in sunlight (c. 3% loss after 12 d continuous irradiation); rapidly photolysed in aqueous solution (DT50 c. 0.33 d).
COMMERCIALISATION
History Discovered by Rhône-Poulenc in 1987. Reported by F. Colliot et al., (Proc. Br. Crop Prot. Conf. - Pests Dis., 1992, 1, 29). Introduced by Rhône-Poulenc Agrochimie (now Bayer CropScience) in 1993. Agricultural uses acquired by BASF AG in 2003. Manufacturers BASF
APPLICATIONS
Biochemistry Insecticide which acts as a potent blocker of the GABA-regulated chloride channel. Insects resistant or tolerant to pyrethroid, cyclodiene, organophosphorus and/or carbamate insecticides are susceptible to fipronil. Mode of action Broad-spectrum insecticide, toxic by contact and ingestion. Moderately systemic and, in some crops, can be used to control insects when applied as a soil or seed treatment. Good to excellent residual control following foliar application. Uses Control of multiple species of thrips on a broad range of crops by foliar, soil or seed treatment. Control of corn rootworm, wireworms and termites by soil treatment in maize. Control of boll weevil and plant bugs on cotton, diamond-back moth on crucifers, Colorado potato beetle on potatoes by foliar application. Control of stem borers, leaf miners, planthoppers, leaf folder/rollers and weevils in rice. Foliar application rates range from 10-80 g/ha; soil treatment rates 100-200 g/ha. Formulation types EC; FS; GR; SC; UL; WG. Selected products: 'Prince' (Nissan)
OTHER PRODUCTS
'Ascend' (BASF); 'Blitz' (BASF); 'Chipco Choice' (BASF); 'Cosmos' (BASF); 'Goliath' (BASF); 'Icon' (BASF); 'KB Guepes' (BASF); 'Metis' (BASF); 'Regent' (BASF); 'Termidor' (BASF); 'Texas' (BASF); 'Violin' (BASF); 'Frontline' (veterinary use) (Bayer CropScience) mixtures: 'Cardinal' (+ aldicarb) (BASF); 'Gazette Prince' (+ carbosulfan) (BASF); 'Jumper' (+ guazatine+ triticonazole) (BASF); 'Regent Plus' (+ aldicarb) (BASF); 'Trident' (+ aldicarb) (BASF); 'Zoom' (+ guazatine+ triticonazole) (BASF); 'Amistar-Prince' (+ azoxystrobin) (rice nursery box) (Syngenta, Nihon Nohyaku, Nissan); 'Delaus-Prince' (+ diclocymet) (Nissan); 'Dr. Oryze-Prince' (+ probenazole) (Meiji Seika, Hokko); 'Fuji-one Prince' (+ isoprothiolane) (Nihon Nohyaku); 'Pika Pika' (+ isoprothiolane+ pyroquilon) (Nihon Nohyaku); 'Vget Prince' (+ tiadinil) (Nihon Nohyaku)
ANALYSIS
Product by hplc with u.v. detection. Residues by glc with ECD.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 89, 91 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats 97, mice 95 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000, rabbits 354 mg/kg. Not a skin or eye irritant (OECD criteria). Not a skin sensitiser. Inhalation LC50 (4 h) for rats 0.682 mg/l (tech.; nose only exposure). NOEL (2 y) for rats 0.5 mg/kg diet; (18 mo) for mice 0.5 mg/kg diet; (52 w) for dogs 0.2 mg/kg b.w. daily (combined sexes). ADI (JMPR) 0.0002 mg/kg b.w. [2000]; group ADI for fipronil and fipronil desulfinyl. Other Non-mutagenic, non-teratogenic; no adverse effect on reproductive performance. Clinical signs of toxicity consistent with the interaction of the molecule at a neurotransmitter receptor were observed in all species tested, but were completely reversible. Toxicity class WHO (a.i.) II; EPA (formulation) II
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail 11.3, mallard ducks >2000, pheasants 31, red-legged partridges 34, house sparrows 1120, pigeons >2000 mg/kg. Dietary LC50 (5 d) for bobwhite quail 49, mallard ducks >5000 mg/kg diet. Fish Acute LC50 (96 h) for bluegill sunfish 85, rainbow trout 248, European carp 430 mg/l. Daphnia LC50 (48 h) 0.19 mg/l; for D. carinata (48 h) 3.8 mg/l. Algae EC50 (96 h) for Scenedesmus subspicatus 0.068 mg/l; (120 h) for Selenastrum capricornutum >0.16, Anabaena flos-aquae >0.17 mg/l. Bees Highly toxic to honeybees, both by direct contact and by ingestion. However, no risk to bees when used as a soil or seed treatment. Worms Non-toxic.
ENVIRONMENTAL FATE
In plants, animals and the environment, fipronil is metabolised via reduction to the sulfide, oxidation to the sulfone, and hydrolysis to the amide. In the presence of sunlight, a photodegradate also forms via sulfoxide extrusion. The sulfide, sulfone and photodegradate are known to act at the GABA receptor site, whereas the amide does not. Animals In rats, once absorbed, the distribution and metabolism of fipronil is rapid. Elimination is mainly via the faeces as fipronil and its sulfone. The two major urinary metabolites were identified as conjugates of ring-opened pyrazole products. The distribution of radioactive residues in tissues was extensive after seven days. In goats and hens, the sulfone was the only metabolite identified in tissues. Plants When applied as an incorporated soil treatment to cotton, maize, sugar beet or sunflowers, uptake of fipronil into plants in all cases was low (c. 5%). At crop maturity, the major residue components observed in all plants were fipronil, the sulfone, and the amide. Following foliar application to cotton, cabbage, rice and potatoes, at crop maturity, fipronil and the photodegradate were the major residue components. Soil/Environment Results of lab. and field studies: Readily degraded: major degradates in soil (aerobic) are sulfone and amide, (anaerobic) are sulfide and amide. Photolysis of soil-applied fipronil gives the photodegradate together with sulfone and amide. Koc 427 (Speyer 2.2) to 1248 (sandy loam). Both fresh and aged column leaching studies (5 soils) indicate that fipronil and its metabolites present a low risk of downward movement in soil; this is supported by field dissipation studies. Following soil incorporated in-furrow granular applications, quantifiable residues were confined to the top 30 cm of soil, with no significant lateral movement or residues.
|