|
fentin
Fungicide
FRAC 30, C6; organotin fungicide
NOMENCLATURE
fentin
Common name fentin (BSI, E-ISO); fentine ((f) F-ISO); fenolovo* (former exception, USSR); chemical name is used (Republic of South Africa, USA)
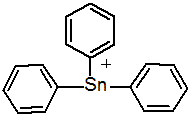
IUPAC name triphenyltin
Chemical Abstracts name triphenylstannylium
CAS RN [668-34-8] Development codes VP 1940; Hoe 02824 (Hoechst)
fentin acetate
IUPAC name triphenyltin(IV) acetate; triphenyltin acetate
Chemical Abstracts name (acetyloxy)triphenylstannane
CAS RN [900-95-8] EEC no. 212-984-0 Development codes Hoe 002782 (Hoechst); AE F002782 (Bayer CropScience) Official codes OMS 1020; ENT 25208
fentin hydroxide
IUPAC name triphenyltin(IV) hydroxide; triphenyltin hydroxide
Chemical Abstracts name hydroxytriphenylstannane
CAS RN [76-87-9] EEC no. 200-990-6 Development codes AE F029664 (Bayer CropScience) Official codes OMS 1017; ENT 28009
PHYSICAL CHEMISTRY
fentin
Mol. wt. 350.0 M.f. C18H15Sn
fentin acetate
Composition Tech. grade is ³94% pure. Mol. wt. 409.0 M.f. C20H18O2Sn Form Colourless crystals. M.p. 121-123 ºC; (tech., 118-125 ºC) V.p. 1.9 mPa (60 ºC) KOW logP = 3.54 Henry 2.96 ´ 10-4 Pa m3 mol-1 (20 °C, BBA method) S.g./density 1.5 (20 ºC) Solubility In water c. 9 mg/l (20 ºC, pH 5). In ethanol 22, ethyl acetate 82, hexane 5, dichloromethane 460, toluene 89 (all in g/l, 20 ºC). Stability Stable when dry. Converted to fentin hydroxide in the presence of water. Unstable in acids and alkalis (22 ºC), DT50 <3 h (pH 5, 7 or 9). Decomposed by sunlight and by atmospheric oxygen. F.p. 185? °C (Cleveland open cup)
fentin hydroxide
Composition Tech. grade is ³95% pure. Mol. wt. 367.0 M.f. C18H16OSn Form Colourless crystals. M.p. 123 °C V.p. 0.0013 mPa (50 ºC) KOW logP = 3.54 Henry 6.28 ´ 10-7 Pa m3 mol-1 (20 °C) S.g./density 1.54 at 20 ºC Solubility In water c. 1 mg/l (pH 7, 20 ºC), (greater at lower pH values). In ethanol 32, isopropanol 48, acetone 46, polyethylene glycol 41 (all in g/l, 20 °C). Stability Stable in the dark at room temperature. Dehydration occurs on heating above 45 ºC, yielding bis(triphenyltin) oxide, which is stable up to c. 250 ºC. Slowly decomposed by sunlight, and more rapidly by u.v. light, to give inorganic tin via di- and mono- phenyltin compounds. F.p. 174 °C (Cleveland open cup)
COMMERCIALISATION
History Fungicidal properties of organotin compounds investigated by G. J. M. van der Kerk & J. G. A. Luijten (J. Appl. Chem., 1954, 4, 314; 1956, 6, 56) and reviewed by H. Bock (Residue Rev., 1981, 79, 1). Fentin acetate was introduced by Hoechst AG (now Bayer CropScience) and fentin hydroxide by N.V. Philips-Duphar (now Crompton Corp.), both in 1959/1960.
fentin acetate
Patents US 3499086 (Hoechst)
APPLICATIONS
fentin acetate
Biochemistry Multi-site inhibitor, preventing spore germination, and inhibiting metabolism of the fungal organism, in particular respiration. Mode of action Non-systemic fungicide with mainly protective action, but also some curative action. Also acts as an algicide and molluscicide. Uses Control of early and late blights of potatoes (at 200-300 g/ha), leaf spot diseases of sugar beet (at 200-300 g/ha), anthracnose of beans (at 200 g/ha). Phytotoxicity Vines, ornamentals, some fruits, and glasshouse crops may be injured. Formulation types WP. Compatibility Incompatible with oil emulsions and EC formulations. Selected products: 'Suzu' (Nihon Nohyaku); mixtures: 'Brestan' (+ maneb) (Bayer CropScience)
fentin hydroxide
Biochemistry Multi-site inhibitor, preventing spore germination, and inhibiting metabolism of the fungal organism, in particular respiration. Mode of action Non-systemic fungicide with protective and curative action. Uses Control of early and late blights of potatoes (at 200-300 g/ha), leaf spot diseases of sugar beet (at 200-300 g/ha), and fungal diseases soya beans (at 200 g/ha). Phytotoxicity Non-phytotoxic when used as directed. Tomatoes and apples may be injured. Surfactants, spreaders or stickers should not be used, as this can lead to phytotoxicity. Formulation types SC; WP. Compatibility Not compatible with strongly acidic compounds. Incompatible with oils and liquid formulations. Selected products: 'Brestan' (Bayer CropScience); 'Super-Tin' (Chiltern, Griffin); 'Suzu-H' (Nihon Nohyaku)
OTHER PRODUCTS
fentin acetate
Discontinued products: 'Fentol' * (Bayer); 'Hokko Suzu' * (Hokko); 'Radar' * (Productos OSA)
fentin hydroxide
'Agri Tin' (Nufarm Ltd); 'Anticercospora' (Siapa); 'Brestanid' (Bayer CropScience); 'Duter' (BASF); 'Farmatin' (Bayer CropScience); 'Keytin' (Chiltern); 'MSS Flotin' (Nufarm UK); 'Rosette' (GreenCrop) mixtures: 'Pro-Tex' (+ maneb) (Griffin); 'Timbal F' (+ tetraconazole) (Sipcam Phyteurop) Discontinued products: 'Du Ter' * (Duphar, AgrEvo); 'Photon' * (Aventis); 'Ashlade Flotin' * (Ashlade) mixtures: 'EndSpray' * (+ metoxuron) (PBI)
ANALYSIS
Product analysis: fentin acetate and hydroxide may be determined by conversion to fentin chloride, followed by rplc with u.v. detection (CIPAC Handbook, 1992, E, 89-94), or by hydrolysis of fentin acetate to the hydroxide, which is measured by potentiometric titration (CIPAC Handbook, 1980, 1A, 1263, 1266; AOAC Methods, 17th Ed., 979.02), or by glc of a derivative (ibid., 984.04; CIPAC Handbook, 1983, 1B, 1837; ibid., 1988, D, 95; Van Rossum et al., Anal. Methods Pestic. Plant Growth Regul., 1980, 11, 227). Residues determined by atomic absorption spectrophotometry of total tin (W. H. Evans et al., Analyst (London), 1979, 104, 16; T. Ferri et al., Talanta, 1989, 36, 513) or by glc of a derivative (H. H. van den Broek et al., Analyst (London), 1988, 113, 1237; Van Rossum, loc. cit.; P. G. Baker et al., Analyst (London), 1980, 105, 282).
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 62, 64 (see part 2 of the Bibliography).
fentin
ADI (JMPR) 0.0005 mg/kg b.w. [1991].
fentin acetate
Oral Acute oral LD50 for rats 140-298 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits 127 mg/kg. Irritating to skin and mucous membranes on repeated application. Inhalation LC50 (4 h) for male rats 0.044, female rats 0.069 mg/l air. NOEL (2 y) for dogs 4 mg/kg diet. ADI 0.0004 mg/kg b.w. Toxicity class WHO (a.i.) II; EPA (formulation) II EC classification R40| R63| T+; R26| T; R24/25, R48/23| Xi; R37/38, R41| N; R50, R53
fentin hydroxide
Oral Acute oral LD50 for rats 150-165 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits 127 mg/kg. Irritating to skin and mucous membranes on repeated application. Inhalation LC50 (4 h) for rats 0.06 mg/l air. NOEL (2 y) for rats 4 mg/kg diet. ADI 0.0004 mg/kg b.w. Toxicity class WHO (a.i.) II; EPA (formulation) II EC classification R40| R63| T+; R26| T; R24/25, R48/23| Xi; R37/38, R41| N; R50, R53
ECOTOXICOLOGY
fentin acetate
Birds LD50 for quail 77.4 mg/kg. Fish LC50 (48 h) for fathead minnow 0.071 mg/l. Daphnia LC50 (48 h) 10 mg/l. Algae LC50 (72 h) 32 mg/l. Bees Formulation not toxic to bees. Worms LD50 (14 d) 128 mg/kg.
fentin hydroxide
Birds Dietary LC50 (8 d) for bobwhite quail 38.5 mg/kg diet. Fish LC50 (48 h) for fathead minnow 0.071 mg/l. Daphnia LC50 (48 h) 10 mg/l. Algae LC50 (72 h) 32 mg/l. Bees Not toxic to bees. Worms LD50 (14 d) 128 mg/kg.
ENVIRONMENTAL FATE
EHC 15 (WHO, 1980). Soil/Environment In soil, fentin acetate and fentin hydroxide are degraded to inorganic tin via di- and mono- phenyltin compounds. Soil DT50 c. 20 d (lab).
|