|
fenthion
Insecticide
IRAC 1B; organophosphate
NOMENCLATURE
Common name fenthion (BSI, E-ISO, (m) F-ISO, ESA, BAN); MPP (JMAF)
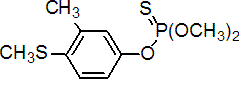
IUPAC name O,O-dimethyl O-4-methylthio-m-tolyl phosphorothioate
Chemical Abstracts name O,O-dimethyl O-[3-methyl-4-(methylthio)phenyl] phosphorothioate
CAS RN [55-38-9] EEC no. 200-231-9 Development codes Bayer 29 493; S 1752; E 1752 Official codes OMS 2; ENT 25 540
PHYSICAL CHEMISTRY
Mol. wt. 278.3 M.f. C10H15O3PS2 Form Colourless, oily liquid; (tech., brown, oily liquid, with a mercaptan-like odour). M.p. No solidification point down to -80 °C B.p. 90 °C/1 Pa (calc.), 117 °C/10 Pa (calc.), 284 °C (calc.) V.p. 0.74 mPa (20 ºC); 1.4 mPa (25 ºC) KOW logP = 4.84 Henry 5 ´ 10-2 Pa m3 mol-1 (20 °C) S.g./density 1.25 (20 ºC) Solubility In water 4.2 mg/l (20 ºC). In dichloromethane, toluene, isopropanol >250, hexane 100 (all in g/l, 20 ºC). Stability Stable to light, and up to 210 ºC. Relatively stable in acidic conditions, and moderately stable in alkaline conditions; DT50 (22 ºC) 223 d (pH 4), 200 d (pH 7), 151 d (pH 9). F.p. 170 ºC (tech.)
COMMERCIALISATION
History Developed by G. Schrader (Hoefchen-Briefe Engl. Ed., 1960, 1, 1) and introduced by Bayer AG in 1960. Patents DE 1116656; US 3042703 Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Cholinesterase inhibitor. Mode of action Insecticide with contact, stomach, and respiratory action. Uses Control of fruit flies, leafhoppers, leaf miners, leaf-eating larvae, stem borers, cereal bugs, and other insect pests in fruit (including citrus), vines, olives, vegetables, cotton, tea, sugar cane, rice, beet, tobacco, ornamentals, etc. For agricultural uses, application rates vary from 60 to 1200 g/ha, depending on crop, pest, pest stage and application method. Control of insect pests (flies, mosquitoes, cockroaches, fleas, ants, ticks, lice, etc.) in public health situations and animal houses, and control of animal ectoparasites. Phytotoxicity Non-phytotoxic when used as recommended. Some varieties of apples and cotton may be injured. Formulation types DP; EC; GR; HN; PO; UL; WP. Compatibility Incompatible with insecticides and fungicides which are highly alkaline. Selected products: 'Faster' (Sanonda); 'Lebaycid' (Bayer CropScience); 'Pilartex' (Pilarquim)
OTHER PRODUCTS
'Baycid' (Bayer CropScience); 'Baytex' (Bayer CropScience) Discontinued products: 'Entex' * (Bayer); 'Tiguvon' * (Bayer); 'Beiliulin' * (Shenzhen Jiangshan)
ANALYSIS
Product analysis by colorimetry of a derivative (F. B. Ibrahim & J. C. Cavagnol, J. Agric. Food Chem., 1966, 44, 369; CIPAC Handbook, 1983, 1B, 1830-1836); details from Bayer CropScience. Residues determined by glc (M. A. Luke et al., J. Assoc. Off. Anal. Chem., 1981, 64, 1187; A. Ambrus et al., ibid., p. 733; Anal. Methods Pestic. Plant Growth Regul., 1972, 6, 301). Methods for the determination of residues are available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 80, 82 (see part 2 of the Bibliography). Oral Acute oral LD50 for male and female rats c. 250 mg/kg. Skin and eye Acute percutaneous LD50 (24 h) for male rats 586, female rats 800 mg/kg; not irritating to skin and eyes (rabbits). Inhalation LC50 (4 h) for male and female rats c. 0.5 mg/l air (aerosol). NOEL (2 y) for rats <5, mice 0.1 mg/kg diet; (1 y) for dogs 2 mg/kg diet. ADI (JMPR) 0.007 mg/kg b.w. [1995, 1997]. Toxicity class WHO (a.i.) II; EPA (formulation) II EC classification R68| T; R23, R48/25| Xn; R21/22| N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail 7.2 mg/kg. Dietary LC50 (5 d) for bobwhite quail 60, mallard ducks 1259 mg/kg. Fish LC50 (96 h) for bluegill sunfish 1.7, rainbow trout 0.83, golden orfe 2.7 mg/l. Daphnia EC50 (48 h) 0.0057 mg/l. Algae ErC50 for Scenedesmus subspicatus 1.79 mg/l. Bees LD50 (contact) 0.16 mg/bee. Worms LC50 for Eisenia foetida 375 mg/kg dry soil.
ENVIRONMENTAL FATE
EHC 63 (WHO, 1986; a general review of organophosphorus insecticides). Animals In mammals, following oral administration, elimination is mainly in the form of hydrolysis products in the urine. Major metabolites are fenthion sulfoxide, fenthion sulfone and their oxygen analogues. These metabolites are hydrolysed in further degradation, forming the corresponding phenols. Plants In plants, fenthion is oxidised to the sulfoxide and sulfone, both of which possess insecticidal properties, and to the monodesmethyl compound of fenthion sulfoxide. Further degradation occurs to the sulfone phosphate, which undergoes hydrolysis. Soil/Environment Koc 1500 (Ware, Rev. Environ. Contam. Toxicol., 123 (1992)). In sediment/water system with and without plants, DT50 is c. 1.5 d (O'Neill et al., Environ. Toxicol. Chem., 8, 759-768 (1989)). The degradation of fenthion occurs rapidly under aerobic conditions, forming the metabolites fenthion sulfoxide, fenthion sulfone, followed by the analogous phenol compounds.
|