|
fenchlorazole-ethyl
Herbicide safener
NOMENCLATURE
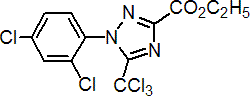
Common name (for acid) fenchlorazole (BSI, draft E-ISO)
IUPAC name ethyl 1-(2,4-dichlorophenyl)-5-trichloromethyl-1H-1,2,4-triazole-3-carboxylate
Chemical Abstracts name ethyl 1-(2,4-dichlorophenyl)-5-(trichloromethyl)-1H-1,2,4-triazole-3-carboxylate
CAS RN [103112-35-2]; [103112-36-3] (acid) Development codes Hoe 070542 (Hoechst); Hoe 072829 (acid)
PHYSICAL CHEMISTRY
Mol. wt. 403.5; (acid 375.4) M.f. C12H8Cl5N3O2; (acid C10H4Cl5N3O2) Form White, practically odourless solid. M.p. 108-112 ºC V.p. 8.9 ´ 10-4 mPa (20 ºC) (OECD 104) S.g./density 1.7 (20 ºC) Solubility In water 0.9 mg/l (pH 4.5, 20 ºC). In n-hexane 2.5, methanol 27, toluene 270, acetone 360, dichloromethane >500 (all in g/l, 20 ºC). Stability Aqueous hydrolysis DT50 115 d (pH 5), 5.5 d (pH 7), 0.079 d (pH 9).
COMMERCIALISATION
History Herbicide safener reported by H. Bieringer et al. (Proc. 1989 Br. Crop Prot. Conf. - Weeds, 1, 77). Fenchlorazole-ethyl introduced by Hoechst AG (now Bayer CropScience). Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Fenchlorazole-ethyl synergises the activity of fenoxaprop-ethyl in susceptible species by enhancing de-esterification to the acid, which is the active form. It also enhances the tolerance of wheat by accelerating the metabolic breakdown of fenoxaprop, leading to the formation of non-phytotoxic degradation products (J. C. Hall & G. R. Stephenson, Proc. Br. Crop Prot. Conf. - Weeds, 1995, 1, 261; J. C. Hall et al., Proc. 9th IUPAC Int. Congr. Pestic. Chem., London, 1998, 5E-005). Mode of action Applied to the foliage of wheat in combination with the grass herbicide fenoxaprop-ethyl or fenoxaprop-P-ethyl, it prevents growth retardation, leaf discolouration, and chlorosis of the crop. Uses Used as a safener for fenoxaprop-ethyl or fenoxaprop-P-ethyl in post-emergence control of grass weeds in wheat, durum wheat, rye, and triticale.
ANALYSIS
Details of hplc method are available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000, mice >2000 mg/kg. Skin and eye Acute percutaneous LD50 for rats and rabbits >2000 mg/kg. Non-irritating to eyes and skin (rabbits). Non-sensitising to skin (guinea pigs). Inhalation LC50 (4 h) >1.52 mg/l. NOEL (90 d) for rats 1280, male mice 80, female mice 320, dogs 80 mg/kg diet; (1 y) for dogs 80 mg/kg diet. Toxicity class WHO (a.i.) U
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >2400 mg/kg. Fish LC50 (96 h) for rainbow trout 0.08 mg/l. Daphnia LC50 (48 h) 1.8 mg/l. Bees Not harmful to bees: LC50 (48 h) >300 mg/bee.
|