|
fenoxaprop-P-ethyl
Herbicide
HRAC A WSSA 1; aryloxyphenoxypropionate
NOMENCLATURE
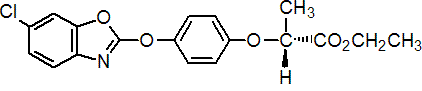
fenoxaprop-P-ethyl
Chemical Abstracts name ethyl (R)-2-[4-[(6-chloro-2-benzoxazolyl)oxy]phenoxy]propanoate
CAS RN [71283-80-2] Development codes Hoe 046360 (Hoechst); AE F046360 (AgrEvo)
fenoxaprop-P
Common name fenoxaprop-P (BSI, draft E-ISO, (m) draft F-ISO)
IUPAC name (R)-2-[4-(6-chloro-1,3-benzoxazol-2-yloxy)phenoxy]propionic acid; (R)-2-[4-(6-chlorobenzoxazol-2-yloxy)phenoxy]propionic acid
Chemical Abstracts name (R)-2-[4-[(6-chloro-2-benzoxazolyl)oxy]phenoxy]propanoic acid
CAS RN [113158-40-0] Development codes Hoe 088406 (Hoechst); AE F088406 (AgrEvo)
PHYSICAL CHEMISTRY
fenoxaprop-P-ethyl
Mol. wt. 361.8 M.f. C18H16ClNO5 Form White, odourless solid. M.p. 89-91 ºC V.p. 5.3 ´ 10-4 mPa (20 ºC) KOW logP = 4.58 Henry 2.74 ´ 10-4 Pa m3 mol-1 (calc.) S.g./density 1.3 (20 °C) Solubility In water 0.7 mg/l (pH 5.8, 20 ºC). In acetone 200, toluene 200, ethyl acetate >200, ethanol c. 24 (all in g/l, 20 ºC). Stability Fenoxaprop-P-ethyl is stable for 90 d at 50 ºC. Not sensitive to light. Decomposed by acids and alkalis, DT50 >1000 d (pH 5), 100 d (pH 7), 2.4 d (pH 9) (20 ºC).
fenoxaprop-P
Mol. wt. 333.7 M.f. C16H12ClNO5 Form Light beige, weakly pungent, fine powder. M.p. 155-161 °C V.p. 1.8 ´ 10-1 mPa (20 °C) KOW logP = 1.83-0.24 (pH 5 - pH 9) S.g./density c. 1.5 (20 °C) Solubility In water 0.27 (pH 5.1), 61 (pH 7.0) (both in g/l, 20 °C). In acetone 80, toluene 0.5, ethyl acetate 36, methanol 34 (all in g/l, 20 °C).
COMMERCIALISATION
History The herbicidal enantiomer of fenoxaprop was reported by H. P. Huff et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1989, 2, 717). Introduced by Hoechst AG (now Bayer CropScience). Manufacturers Bayer CropScience; Sharda; Sundat; Tide
APPLICATIONS
fenoxaprop-P-ethyl
Biochemistry Fatty acid synthesis inhibition in grasses, by inhibition of acetyl CoA carboxylase (ACCase). Mode of action Fenoxaprop-P-ethyl is a selective herbicide with contact and systemic action, absorbed principally by the leaves, with translocation both acropetally and basipetally to the roots or rhizomes. Uses Post-emergence control of annual and perennial grass weeds in potatoes, beans, soya beans, beets, vegetables, peanuts, flax, oilseed rape, and cotton; and (when applied with the herbicide safener mefenpyr-diethyl) annual and perennial grass weeds and wild oats in wheat, rye, triticale and, depending on ratio, in some varieties of barley. Phytotoxicity Non-phytotoxic to broad-leaved crops. Formulation types EC; EW; SE. Selected products: 'Furore Super' (Bayer CropScience); 'Masaldo' (Crop Health); 'Puma' (Bayer CropScience); 'Whip Super' (Bayer CropScience); mixtures: 'Puma Super' (+ mefenpyr-diethyl) (Bayer CropScience)
OTHER PRODUCTS
fenoxaprop-P-ethyl
'Acclaim Super' (Bayer CropScience); 'Bugle' (Bayer CropScience); 'Depon Super' (Bayer CropScience); 'Option II' (Bayer CropScience); 'Ricestar' (with safener) (Bayer CropScience); 'Silverado' (Bayer CropScience); 'Triumph' (Bayer CropScience); 'Whip 360' (Bayer CropScience) mixtures: 'Baghera' (+ mefenpyr-diethyl+ diclofop-methyl) (Bayer CropScience); 'Cheetah Super' (+ mefenpyr-diethyl) (Bayer CropScience); 'Cheyenne' (+ MCPA-2-ethylhexyl) (Bayer CropScience); 'Corniche' (+ diclofop-methyl) (Bayer CropScience); 'Dakota' (+ MCPA-2-ethylhexyl) (Bayer CropScience); 'Djinn' (+ isoproturon+ mefenpyr-diethyl) (Bayer CropScience); 'Dopler' (+ mefenpyr-diethyl+ diclofop-methyl) (Bayer CropScience); 'Fusion' (+ fluazifop-P-butyl) (Syngenta, Bayer CropScience); 'Horizon 2000' (+ fluazifop-P-butyl) (Bayer CropScience); 'Preclaim' (+ pendimethalin) (Bayer CropScience); 'Proper energy' (+ mefenpyr-diethyl) (Bayer CropScience); 'Puma Extra' (+ isoproturon) (Bayer CropScience); 'Puma S' (+ mefenpyr-diethyl) (Bayer CropScience); 'Puma X' (+ isoproturon) (Bayer CropScience); 'Tigress Ultra' (+ diclofop-methyl) (Bayer CropScience); 'Tiller' (+ MCPA-2-ethylhexyl+ 2,4-D-2-ethylhexyl) (Bayer CropScience); 'Twister' (+ fluazifop-P-butyl+ fomesafen-sodium) (Syngenta); 'Zeus' (+ mefenpyr-diethyl+ diclofop-methyl) (Bayer CropScience) Discontinued products mixtures: 'Tigress' * (+ diclofop-methyl) (AgrEvo); 'Tiller' * (+ MCPA-2-ethylhexyl+ 2,4-D-isoctyl) (AgrEvo)
ANALYSIS
Details on hplc method are available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
fenoxaprop-P-ethyl
Oral Acute oral LD50 for rats 3150-4000, mice >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Inhalation LC50 (4 h) for rats >1.224 mg/l air. NOEL (90 d) for rats 0.75 mg/kg b.w. daily (10 ppm), mice 1.4 mg/kg b.w. daily (10 ppm), dogs 15.9 mg/kg b.w. daily (400 ppm).
ECOTOXICOLOGY
fenoxaprop-P-ethyl
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Fish LC50 (96 h) for bluegill sunfish 0.58, rainbow trout 0.46 mg/l. Daphnia LC50 (48 h) 0.56 (pH 8.0-8.4), 2.7 (pH 7.7-7.8) (both mg/l). Algae LC50 (72 h) for Scenedesmus subspicatus 0.51 mg/l. Bees LC50 (contact) >300 mg/bee; (feed) >1000 mg/bee. Worms LC50 (14 d) for Eisenia foetida >1000 mg/kg soil.
ENVIRONMENTAL FATE
Plants In plants, fenoxaprop-ethyl is metabolised via fenoxaprop to 6-chloro-2,3-dihydrobenzoxazol-2-one. Soil/Environment In soil, fenoxaprop-ethyl is rapidly hydrolysed to fenoxaprop (A. E. Smith, J. Agric. Food Chem., 1985, 33, 483); DT50 1-10 d.
|