|
fenamiphos
Nematicide
IRAC 1B; organophosphate
NOMENCLATURE
Common name fenamiphos (BSI, E-ISO); phénamiphos ((m) F-ISO)
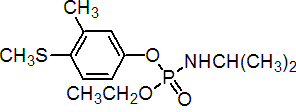
IUPAC name ethyl 4-methylthio-m-tolyl isopropylphosphoramidate
Chemical Abstracts name ethyl 3-methyl-4-(methylthio)phenyl (1-methylethyl)phosphoramidate
Other names methaphenamiphos CAS RN [22224-92-6] EEC no. 244-848-1 Development codes BAY 68 138 (Bayer); SRA 3886
PHYSICAL CHEMISTRY
Mol. wt. 303.4 M.f. C13H22NO3PS Form Colourless crystals; (tech., tan, waxy solid). M.p. 49.2 ºC; (tech., 46 ºC) V.p. 0.12 mPa (20 ºC); 4.8 mPa (50 ºC) KOW logP = 3.30 (20 °C) Henry 9.1 ´ 10-5 Pa m3 mol-1 (20 °C) S.g./density 1.191 (23 ºC) Solubility In water 0.4 g/l (20 ºC). In dichloromethane, isopropanol, toluene >200, hexane 10-20 (all in g/l, 20 ºC). Stability Hydrolysis DT50 1 y (pH 4), 8 y (pH 7), 3 y (pH 9) (22 ºC). F.p. c. 200 °C
COMMERCIALISATION
History Nematicide reported by J. H. O'Bannon & A. L. Taylor (Plant Dis. Rep., 1967, 51, 995) and B. Homeyer (Pflanzenschutz-Nachr. (Engl. Ed.), 1971, 24, 48). Introduced by Baychem Corp., Chemagro Division, in 1968. Patents DE 1121882; US 2978479
APPLICATIONS
Biochemistry Cholinesterase inhibitor. Mode of action Systemic nematicide with contact action. Absorbed by the roots, with translocation to the leaves. Against soil populations of ectoparasitic nematodes, fenamiphos is more persistent and more effective than the sulfoxide metabolite; the sulfone metabolite is least effective. Against plant endoparasitic nematodes, the sulfone is more effective than the sulfoxide (T. B. Waggoner & A. M. Khasawinah (Residue Rev., 1974, 53, 79)). Uses Control of ectoparasitic, endoparasitic, free-living, cyst-forming, and root-knot nematodes. Used in bananas, pineapples, citrus fruit, pome fruit, stone fruit, vines, hops, cotton, cocoa, coffee, okra, peanuts, soya beans, cucurbits, tomatoes, potatoes, vegetables, sugar beet, ornamentals, tobacco, and turf. Has secondary activity against sucking insects and spider mites. Phytotoxicity Non-phytotoxic when applied to the soil. Formulation types EC; EW; GR. Compatibility Incompatible with alkaline materials. Selected products: 'Nemacur' (Bayer CropScience, Makhteshim-Agan)
OTHER PRODUCTS
'Fenami' (Makhteshim-Agan, Bayer CropScience) mixtures: 'Bayfidan Triple' (+ triadimenol+ disulfoton) (Bayer CropScience)
ANALYSIS
Product analysis by glc; details available from Bayer CropScience. Residue analysis by glc (M. A. Luke et al., J. Assoc. Off. Anal. Chem., 1981, 64, 1187; Man. Pestic. Residue Anal., 1987, I, 6, S16, S19; Anal. Methods Residues Pestic., 1988, Part I, M5, M12; A. R. C. Hill et al., Analyst (London), 1984, 109, 483). In drinking water, by glc with NPD (AOAC Methods, 17th Ed., 991.07); fenamiphos sulfoxide and sulfone can be determined by lc with u.v. detection (AOAC Methods, 17th Ed., 992.14). Details available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 80, 82 (see part 2 of the Bibliography). Oral Acute oral LD50 for male and female rats c. 6, mice, dogs and cats c. 10 mg/kg. Skin and eye Acute percutaneous LD50 for rats c. 80 mg/kg. Slightly irritant to skin and eyes (rabbits). Inhalation LC50 (4 h) for rats c. 0.12 mg/l air (aerosol). NOEL (2 y) for rats 1, mice 10 mg/kg diet; (12 mo) for dogs 1 mg/kg diet. ADI (JMPR) 0.0008 mg/kg b.w. [1997]. Toxicity class WHO (a.i.) Ib; EPA (formulation) I EC classification T+; R28| T; R24
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail 0.7-1.6, mallard ducks 0.9-1.2 mg/kg. Dietary LC50 (5 d) for mallard ducks 316, bobwhite quail 38 mg/kg diet. Fish LC50 (96 h) for bluegill sunfish 0.0096, rainbow trout 0.0721 mg/l. Daphnia LC50 (48 h) 0.0019 mg/l. Algae ErC50 for Scenedesmus subspicatus 11 mg/l. Worms LC50 for Eisenia foetida 795 mg/kg dry soil (400 EC).
ENVIRONMENTAL FATE
EHC 63 (WHO, 1986; a general review of organophosphorus insecticides). Animals In mammals, following oral administration, there is rapid metabolism involving oxidation to the sulfoxide and sulfone analogues, followed by subsequent hydrolysis, conjugation and excretion via the urine; some N-dealkylation also occurs (T. B. Waggoner & A. M. Khasawinah, Residue Rev., 1974, 53, 79). Plants Degradation is by thiooxidation and hydrolysis. The major metabolites are fenamiphos sulfoxide and fenamiphos sulfone (idem, ibid.). Soil/Environment No effect on soil bacteria. Readily degradable in water, degradable on soil surfaces. Duration of activity in soil is c. 4 months. Based on Koc values and leaching studies, fenamiphos can be classified as a compound with low mobility. Soil DT50 (aerobic and anaerobic) several weeks. The major degradation products are fenamiphos sulfoxide and fenamiphos sulfone and their phenols.
|