|
famoxadone
Fungicide
FRAC 11, C3; strobilurin type: oxazolidinedione
NOMENCLATURE
Common name famoxadone (BSI, pa ISO, ANSI)
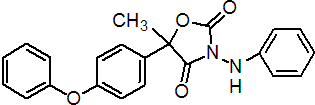
IUPAC name 3-anilino-5-methyl-5-(4-phenoxyphenyl)-1,3-oxazolidine-2,4-dione
Chemical Abstracts name 5-methyl-5-(4-phenoxyphenyl)-3-(phenylamino)-2,4-oxazolidinedione
CAS RN [131807-57-3] Development codes JE874; DPX-JE874; IN-JE874 (all DuPont)
PHYSICAL CHEMISTRY
Composition Racemic. Mol. wt. 374.4 M.f. C22H18N2O4 M.p. 141.3-142.3 °C V.p. 6.4 ´ 10-4 mPa (20 °C) KOW logP = 4.65 (pH 7) Henry 4.61 ´ 10-3 Pa m3 mol-1 (calc., 20 °C) S.g./density 1.31 (22 °C) Solubility In water 52 ppb (unbuffered water pH 7.8-8.9, 20 °C). Stability Solid, tech. famoxadone stable in the dark 14 d at 25 °C or 54 °C. In water with no light, DT50 41 d (pH 5), 2 d (pH 7), 0.0646 d (pH 9) (25 °C); in water with light, DT50 4.6 d (pH 5, 25 °C). pKa Does not dissociate
COMMERCIALISATION
History Reported by M. M. Joshi & J. A. Sternberg (Proc. Br. Crop Prot. Conf. - Pests Dis., 1996, 1, 21). Introduced in 1998 by E. I. du Pont de Nemours and Co. Manufacturers DuPont
APPLICATIONS
Biochemistry Inhibits mitochondrial electron transport, by blocking ubiquinol:cytochrome c oxidoreductase at complex III. Activity resides mainly in the (S)- isomer. Mode of action Protectant and residual fungicide. Acts mainly by inhibiting spore germination. Uses Control of a broad spectrum of plant pathogenic fungi, at 50-200 g/ha. Particularly effective against grape downy mildew, potato and tomato late and early blights, downy mildew of cucurbits, wheat leaf and glume blotch, and barley net blotch. Formulation types WG; EC. Selected products: mixtures: 'Equation Contact' (+ mancozeb) (DuPont); 'Equation Pro' (+ cymoxanil) (DuPont); 'Tanos' (+ cymoxanil) (DuPont)
OTHER PRODUCTS
'Famoxate' (DuPont) mixtures: 'Charisma' (+ flusilazole) (DuPont); 'Clip' (+ mancozeb) (DuPont); 'Equation R' (+ copper sulfate) (DuPont); 'Equation System' (+ fosetyl-aluminium) (DuPont); 'Horizon' (+ cymoxanil) (DuPont, Nissan); 'Impresario' (+ fosetyl-aluminium) (DuPont); 'Iteral' (+ cymoxanil) (DuPont); 'Midas' (+ mancozeb) (DuPont)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Not a skin or eye irritant (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >5.3 mg/l. NOEL for male rats 1.62, female rats 2.15, male mice 95.6, female mice 130, male dogs 1.2, female dogs 1.2 mg/kg b.w. daily. ADI 0.012 mg/kg b.w. Other Neither a reproductive or developmental toxin, negative for neurotoxicity in acute and subchronic studies, not an oncogen, and does not pose a genotoxic hazard. Toxicity class WHO (a.i.) III (company assignment); EPA (formulation) III
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2250 mg/kg. Dietary LC50 (5 d) for bobwhite quail and mallard ducks >5260 mg/kg. Fish LC50 (96 h) for rainbow trout 0.011, sheepshead minnow 0.049, carp 0.17 mg/l. Daphnia EC50 (48 h) 0.012 mg/l. Algae EbC50 (72 h) for Selenastrum capricornutum 0.022 mg/l. Other aquatic spp. LC50 (96 h) for Mysidopsis bahia, 0.0039 mg/l; EC50 (96 h, shell deposition) for Crassostrea virginica 0.0014 mg/l. Bees LD50 for honeybees >25 mg/bee; LC50 (48 h) >1000 ppm. Worms LC50 (14 d) for earthworms 470 mg/kg soil.
ENVIRONMENTAL FATE
Animals Following oral application to rats, elimination is rapid. Unmetabolised famoxadone was the major component in the faeces; mono- (at 4'-phenoxyphenyl) and di- (also at 4-phenylamino) hydroxylated famoxadone were the primary faecal metabolites. In urine, products arising from cleavage of the heterocyclic ring were found (M. C. Savides et al., Proc. 9th IUPAC Int. Congr. Pestic. Chem., London, 1998,2, 5B-003). In goats and hens, there was little residue in the tissues; the majority of the administered famoxadone (c. 60%) was unmetabolised and recovered in the faeces. Metabolism was complex, involving hydroxylation, cleavage of the oxazolidinedione-aminophenyl linkage, cleavage of the phenoxyphenyl ether linkage and opening of the oxazolidinedione ring (D. Y. Lee et al., ibid., 5B-004). Plants In grapes, tomatoes and potatoes, famoxadone was the main residue; no residues were found in potato tubers. In wheat, famoxadone was more extensively metabolised, primarily by hydroxylation, followed by conjugation. Soil/Environment In laboratory soil, DT50 6 d (aerobic, 20 °C, 40-50% MWHC, pH 5.3-8.0, 1.1-2.9% o.m.), 28 d (anaerobic, 20 °C, pH 7.2, 1.4% o.m.). Degradation routes include hydroxylation (at the 4'-phenoxyphenyl position), ring opening (with formation of a glycolic acid derivative), and is primarily microbial; it is accelerated by light (K. M. Jernberg et al., ibid., 6A-009).
|