|
etoxazole
Acaricide
IRAC 10B; mite growth inhibitor
NOMENCLATURE
Common name etoxazole (BSI, pa ISO)
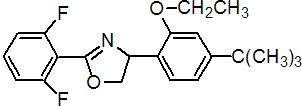
IUPAC name (RS)-5-tert-butyl-2-[2-(2,6-difluorophenyl)-4,5-dihydro-1,3-oxazol-4-yl]phenetole
Chemical Abstracts name 2-(2,6-difluorophenyl)-4-[4-(1,1-dimethylethyl)-2-ethoxyphenyl]-4,5-dihydrooxazole
CAS RN [153233-91-1] Development codes YI-5301 (Yashima); S-1283 (Sumitomo)
PHYSICAL CHEMISTRY
Composition Tech. is 93-98%. Mol. wt. 359.4 M.f. C21H23F2NO2 Form White, crystalline powder. M.p. 101-102 ºC V.p. 2.18 ´ 10-3 mPa (25 ºC) KOW logP = 5.59 (25 ºC) S.g./density 1.24 (20 °C) Solubility In water 75.4 mg/l (20 ºC). In acetone 300, methanol 90, ethanol 90, cyclohexanone 500, tetrahydrofuran 750, acetonitrile 80, ethyl acetate 250, xylene 250, n-hexane 13, n-heptane 13 (all in g/l, 20 ºC). Stability No decomposition after 30 d (50 ºC). Stable in alkali. F.p. 457 °C (ASTM E 659)
COMMERCIALISATION
History Discovered by Yashima Chemical Industry Co., Ltd and reported by T. Ishida et al. (Proc. Br. Crop Prot. Conf. - Pests Dis., 1994, 1, 37). Registered in Japan in 1998. Under joint development by Yashima and Sumitomo Chemical Co., Ltd. Patents EP 0639572 B1, US 5478855, JP 3189011 Manufacturers Yashima
APPLICATIONS
Mode of action Contact acaricide. Appears to inhibit moulting of mites and aphids. Uses Non-systemic acaricide with effect on eggs, larvae and nymphs, with no effect on adults. Controls many phytophagous mites (Tetranychus, Panonychus spp.) in citrus, pome fruit, vegetables and strawberries, at 50 g/ha, and in tea, at 100 g/ha. Formulation types SC; Smoking agent. Compatibility Cannot be mixed with Bordeaux mixture. Selected products: 'Baroque' (Yashima); mixtures: 'Biruku' (+ fenpropathrin) (Yashima, Sumitomo)
OTHER PRODUCTS
'Borneo' (Philagro); 'Secure' (Valent Biosciences); 'Zoom' (Valent) mixtures: 'Virk' (+ fenpropathrin) (Yashima, Sumitomo)
ANALYSIS
Product and residue analysis by hplc.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male and female rats and mice >5000 mg/kg. Skin and eye Acute percutaneous LD50 for male and female rats >2000 mg/kg; no skin or eye irritation (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 for male and female rats >1.09 mg/l. NOEL NOEL for rats 4.01 mg/kg b.w. daily. ADI 0.04 mg/kg b.w. Other Negative in Ames test.
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >2000 mg/kg. Sub-acute oral LD50 (5 d) for bobwhite quail >5200 ppm diet. Fish LC50 (96 h) for Japanese carp 0.89 mg/l; (48 h) for Japanese carp >20, rainbow trout >40 ppm. Daphnia LC50 (3 h) >40 ppm. Algae EC50 for Selenastrum capricornutum >1.0 mg/l. Other aquatic spp. Disruption of moulting was observed in aquatic arthropods. Bees LD50 (oral and contact) >200 mg/bee. Worms NOEL (14 d) for Eisenia foetida >1000 ppm.
ENVIRONMENTAL FATE
Soil/Environment In Japanese alluvial soil, DT50 19 d, DT90 90 d.
|