|
ethoxysulfuron
Herbicide
HRAC B WSSA 2; sulfonylurea
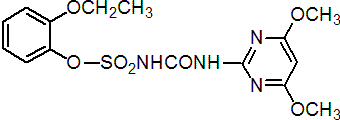
NOMENCLATURE
Common name ethoxysulfuron (BSI, pa ISO)
IUPAC name 1-(4,6-dimethoxypyrimidin-2-yl)-3-(2-ethoxyphenoxysulfonyl)urea
Chemical Abstracts name 2-ethoxyphenyl [[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]sulfamate
CAS RN [126801-58-9] Development codes Hoe 095404 (Hoechst); Hoe-404 (Hoechst)
PHYSICAL CHEMISTRY
Mol. wt. 398.4 M.f. C15H18N4O7S Form White to beige powder. M.p. 144-147 °C V.p. 6.6 ´ 10-2 mPa KOW logP = 2.89 (pH 3), 0.004 (pH 7), -1.2 (pH 9) (20 °C) Henry (calc.) 1.00 ´ 10-3 (pH 5); 1.94 ´ 10-5 (pH 7); 2.73 ´ 10-6 (pH 9) Pa m3 mol-1 (20 °C) Solubility In water 26 (pH 5), 1353 (pH 7), 9628 (pH 9) ppm (20 °C). Stability Hydrolytic DT50 65 d (pH 5), 259 d (pH 7), 331 d (pH 9).
COMMERCIALISATION
History Reported by E. Hacker et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1995, 1, 73). Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor. Acts by inhibiting biosynthesis of the essential amino acids valine and isoleucine, hence stopping cell division and plant growth. Selectivity is due to differential metabolism in crop and weed (H Köcher & G Dickerhof, Proc. Br. Crop Prot. Conf. - Weeds, 1995, 1, 249). Metabolic basis of crop selectivity in sulfonylureas reviewed (M. K. Koeppe & H. M. Brown, Agro-Food-Industry, 6, 9-14 (1995)). Uses Under development for broad-leaved and sedge weed control in cereals, rice and sugar cane, at 10-120 g/ha. Formulation types WG.
OTHER PRODUCTS
'Sunrice' (Bayer CropScience); 'Sunrise' (Bayer CropScience); 'Sunstar' (Bayer CropScience) mixtures: 'Pulgman' (+ pretilachlor+ daimuron) (Bayer CropScience); 'Sanattack' (+ cafenstrole) (Sankyo Agro); 'Topran' (+ pretilachlor+ pyrazolynate) (Bayer CropScience) Discontinued products mixtures: 'Bingo' * (+ anilofos+ benfuresate+ daimuron) (Aventis); 'Goku-Jumbo' * (+ anilofos+ daimuron) (Aventis); 'Kimanmae' * (+ anilofos+ daimuron) (Aventis); 'Kita-bingo' * (+ anilofos+ benfuresate) (Aventis)
ANALYSIS
Methods for sulfonylurea residues in crops, soil and water reviewed (A. C. Barefoot et al., Proc. Br. Crop Prot. Conf. - Weeds, 1995, 2, 707).
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >3270 mg/kg. Skin and eye Acute percutaneous LD50 for rats <4000 mg/kg. Not irritating to eyes or skin (rats). Other Not mutagenic (Ames). EC classification N; R50, R53
ENVIRONMENTAL FATE
Soil/Environment In lab. tests, DT50 in biologically active soil is c. 18-20 d. Under paddy conditions, DT50 is 10-60 d.
|