|
epoxiconazole
Fungicide
FRAC 3, G1; DMI: triazole
NOMENCLATURE
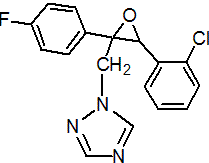
Common name epoxiconazole (BSI, pa ISO)
IUPAC name (2RS,3SR)-1-[3-(2-chlorophenyl)-2,3-epoxy-2-(4-fluorophenyl)propyl]-1H-1,2,4-triazole
Chemical Abstracts name cis-1-[[3-(2-chlorophenyl)-2-(4-fluorophenyl)oxiranyl]methyl]-1H-1,2,4-triazole
CAS RN [106325-08-0] EEC no. 406-850-2 Development codes BAS 480F (BASF)
PHYSICAL CHEMISTRY
Composition Material is the 2R,3S- 2S,3R- enantiomer pair. Mol. wt. 329.8 M.f. C17H13ClFN3O Form Colourless crystals. M.p. 136.2 ºC V.p. <0.01 mPa (20 ºC) KOW logP = 3.44 (pH 7) Henry <4.71 ´ 10-4 Pa m3 mol-1 (calc.) S.g./density 1.384 (room temperature) Solubility In water 6.63 ´ 10-4 g/100 ml (20 ºC). In acetone 14.4, dichloromethane 29.1, heptane 0.04 (all in g/100 ml). Stability No hydrolysis at pH 5 and pH 7 within 12 days.
COMMERCIALISATION
History Developed and introduced by BASF AG; first registrations in 1993. Patents EP 94564; US 4464381 Manufacturers BASF
APPLICATIONS
Biochemistry Inhibition of C-14-demethylase in sterol biosynthesis. Mode of action Preventive and curative fungicide. Uses Broad-spectrum fungicide, with preventive and curative action, for control of diseases caused by Ascomycetes, Basidiomycetes, and Deuteromycetes in cereals, sugar beet, peanuts, oilseed rape, and ornamentals, generally at 125 g/ha. Formulation types SC; SE. Compatibility Compatible with morpholines and MBC-derivatives. Selected products: 'Opus' (BASF); 'Soprano' (Makhteshim-Agan); mixtures: 'Allegro' (+ kresoxim-methyl) (BASF); 'Opus Team' (+ fenpropimorph) (BASF)
OTHER PRODUCTS
'Swing' (BASF) mixtures: 'Duett' (+ carbendazim) (BASF); 'Eclipse' (+ fenpropimorph) (BASF); 'Juwel' (+ kresoxim-methyl) (BASF); 'Landmark' (+ kresoxim-methyl) (BASF); 'Mantra' (+ fenpropimorph+ kresoxim-methyl) (BASF); 'Ogam' (+ kresoxim-methyl) (BASF); 'Opera' (+ pyraclostrobin) (BASF); 'Opponent' (+ kresoxim-methyl+ pyraclostrobin) (BASF); 'Opus Forte' (+ tridemorph) (BASF); 'Opus Plus' (+ tridemorph) (BASF); 'Rex' (+ thiophanate-methyl) (BASF); 'Swing Gold' (+ dimoxystrobin) (BASF); 'Tango Duo' (+ tridemorph) (BASF); 'Avalon' (+ kresoxim-methyl) (Barclay); 'Capricorn' (+ carbendazim) (Bayer CropScience); 'Epoxifen' (+ fenpropimorph) (Standon); 'Galore' (+ fenpropimorph) (GreenCrop); 'KME' (+ kresoxim-methyl) (Me2); 'Landgold Strobilurin KE' (+ kresoxim-methyl) (Landgold); 'Riverdance' (+ fenpropimorph) (Barclay); 'Soprano C' (+ carbendazim) (Makhteshim-Agan) Discontinued products: 'Epic' * (BASF)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to eyes and skin of rabbits. Inhalation LC50 (4 h) for rats >5.3 mg/l air. NOEL (carcinogenicity) for mice 0.81 mg/kg b.w. ADI 0.0032 mg/kg. EC classification R40| R61| R62| N; R51, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for quail >2000 mg/kg. LC50 for quail 5000 mg/kg. Fish LC50 (96 h) for trout 2.2-4.6, bluegill sunfish 4.6-6.8 mg/kg. Daphnia LC50 (48 h) 8.7 mg/l. Algae EC50 (72 h) for green algae 2.3 mg/l. Bees LD50 >100 mg/bee. Worms EC50 (14 d) >1000 mg/kg soil.
ENVIRONMENTAL FATE
Animals A.i. is readily excreted via faeces. There are no major metabolites, but a high number of minor metabolites was identified. The important metabolic reactions were cleavage of the oxirane ring, hydroxylation of the phenyl rings and conjugation. Plants There is extensive degradation. Soil/Environment Degradation in soil is by microbial activity, DT50 c. 2-3 mo. Koc 957-2647.
|