|
endothal
Herbicide, algicide, plant growth regulator
NOMENCLATURE
Common name endothal (BSI, France, New Zealand; since 1990 E-ISO, (m) F-ISO); endothall (ANSI, Canada, WSSA)
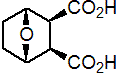
IUPAC name 7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylic acid
Chemical Abstracts name 7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylic acid
Other names 1,2-dicarboxy-3,6-endo-cyclohexane; 3,6-endoxohexahydrophthalic acid CAS RN [145-73-3] endothal, unstated stereochemistry; [28874-46-6] endothal rel-(1R,2S,3R,4S)- isomer; [129-67-9] endothal-sodium, unstated stereochemistry; [17439-94-0] endothal-diammonium, unstated stereochemistry EEC no. 205-660-5; 204-959-8 (sodium salt)
PHYSICAL CHEMISTRY
Composition Of the 4 theoretical stereoisomers of endothal, the rel-(1R,2S,3R,4S)- isomer is the most effective herbicide (US 2550494). Mol. wt. 186.2 M.f. C8H10O5 Form Colourless crystals (monohydrate). M.p. 144 ºC (monohydrate) V.p. 2.09 ´ 10-5 mPa (24.3 °C) KOW logP = -2.09 (unstated pH) Henry 3.8 ´ 10-13 Pa m3 mol-1 (calc.) S.g./density 1.431 at 20 ºC Solubility In water 100 g/kg (20 ºC). In methanol 280, dioxane 76, acetone 70, isopropanol 17, diethyl ether 1, benzene 0.1 (all in g/kg, 20 ºC). Stability Stable to light. Stable up to c. 90 ºC, above which it undergoes a slow conversion to the anhydride. Endothal is a dibasic acid, and forms water-soluble amine and alkali-metal salts. pKa A dibasic acid (pKa1 3.4, pKa2 6.7) F.p. Non-flammable
COMMERCIALISATION
History Herbicide reported by N. Tischler et al. (Proc. Northeast. Weed Control Conf., 1951, p. 51). Introduced by Sharples Chemical Corp. (now Cerexagri Inc.). Patents US 2550494; US 2576080; US 2576081 Manufacturers Cerexagri
APPLICATIONS
Mode of action Selective contact herbicide, absorbed by the leaves and roots, with limited acropetal translocation in the xylem. Also has algicidal properties, and acts as a defoliant and desiccant. Uses Amine, ammonium and alkali-metal salts of endothal are used pre- and post-emergence for control of annual grass and broad-leaved weeds in sugar beet, fodder beet, beetroot, spinach, and turf, at 2-6 kg/ha. Control of algae and aquatic weeds (including use in rice). Also used as a desiccant for alfalfa, clover, and hops; for defoliation of cotton (as a harvest aid); for destruction of potato haulms, Formulation types GR; SL. Selected products: 'Accelerate' (mixture of mono- and di- (N,N-dimethylalkylammonium) salts ('alkyl' being from coconut oil)) (Cerexagri); 'Herbicide 273' (dipotassium salt) (Cerexagri)
OTHER PRODUCTS
'Aquathol' (dipotassium salt) (Cerexagri); 'Desicate II' (mono-(N,N-dimethylalkylammonium) salt) (Cerexagri); 'Des-I-Cate' (mixture of mono- and di- (N,N-dimethylalkylammonium) salts ('alkyl' being from coconut oil)) (Cerexagri); 'Evac' (mono (N,N)-dimethylalkylamine salt (alkyl is C8-C18)) (Cerexagri); 'Hydrothol' (mono-(N,N-dimethylalkylammonium) salt) (Cerexagri) Discontinued products: 'Pennout' * (sodium salt) (Hortichem)
ANALYSIS
Product analysis by glc with FID (R. Carlson et al., Anal. Methods Pestic. Plant Growth Regul., 1978, 10, 327). Residues determined by glc of a derivative, which is measured by MCD (idem, ibid.). Details available from Cerexagri.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 38-54 mg/kg (acid), 206 mg/kg (66.7% formulation of the amine salt). Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/l (acid). Inhalation LC50 (14 d) 0.68 mg/l (acid). NOEL In 2 y feeding trials, rats receiving 1000 mg/kg diet showed no ill-effects. Toxicity class EPA (formulation) II EC classification T; R25| Xn; R21| Xi; R36/37/38 (also assigned to sodium salt)
ECOTOXICOLOGY
Birds Acute LD50 for mallard ducks 111 mg/kg b.w. Dietary LC50 (8 d) for bobwhite quail and mallard ducks >5000 ppm. Fish LC50 (96 h) for trout 49, bluegill sunfish 77 mg/l. Daphnia LC50 (48 h) 92 mg/l. Algae Toxic to algae. Other aquatic spp. LC50 (96 h) for Eastern oysters 54, mysid shrimp 39, fiddler crab 85.1 mg/l. Bees Not toxic to bees.
ENVIRONMENTAL FATE
Animals Rapidly absorbed. Elimination DT50 1.8-2.5 h. Plants Residues in the plant were mainly endothal. Soil/Environment DT50 in aerobic soil 8.5 d. Kd 1.3-37.1. For a review, see Simsiman et al. (Residue Rev., 1976, 62, 131-174).
|