|
dodemorph
Fungicide
FRAC 5, G2; morpholine: morpholine
NOMENCLATURE
dodemorph
Common name dodemorph (BSI, E-ISO); dodémorphe ((m) F-ISO)
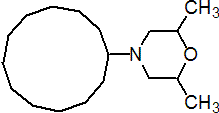
IUPAC name 4-cyclododecyl-2,6-dimethylmorpholine
Chemical Abstracts name 4-cyclododecyl-2,6-dimethylmorpholine
CAS RN [1593-77-7]; dodemorph was originally reported in the literature with an incorrect structure [35842-17-2] EEC no. 216-474-9
dodemorph acetate
Common name dodemorph acetate (BSI, E-ISO); acétate de dodémorphe (F-ISO)
IUPAC name 4-cyclododecyl-2,6-dimethylmorpholinium acetate
CAS RN [31717-87-0] Development codes BAS 238F (BASF)
PHYSICAL CHEMISTRY
dodemorph
Composition Dodemorph contains cis- (c. 60% m/m) and trans- (c. 40%) isomers. Mol. wt. 281.5 M.f. C18H35NO Form The trans- isomer is a colourless oil. The cis- isomer is a colourless solid, with characteristic odour. M.p. 71 ºC B.p. c. 190 ºC/1 mmHg V.p. cis- isomer: 0.48 mPa (20 ºC) KOW logP = 4.14 (pH 7) Solubility cis- isomer: In water <100 mg/kg. In chloroform >1000, ethanol 50, acetone 57, ethyl acetate 185 (all in g/l, 20 ºC). Stability Stable to heat, light and water. pKa 8.08
dodemorph acetate
Composition Dodemorph acetate contains cis- (c. 50-60% m/m) and trans- (c. 50-40%) isomers; tech. has a nominal content of 96% a.i. Mol. wt. 341.5 M.f. C20H39NO3 Form Colourless solid; (tech., viscous yellow liquid). M.p. 63-64 ºC; cis- isomer 72 ºC B.p. 315 °C/101.3 kPa V.p. 12 mPa (25 ºC, gas saturation method) KOW logP = 2.52 (pH 5), 4.23 (pH 9) Henry 0.008 Pa m3 mol-1 (calc.) S.g./density c. 0.93 Solubility In water 736 (pH 5), 520 (pH 7), 2.29 (pH 9) (all in mg/l, 25 ºC). In benzene, chloroform >1000, cyclohexane 846, ethyl acetate 205, ethanol 66, acetone 22 (all in g/kg, 20 ºC). Stability Stable in unopened containers for >1 y. Stable at 50 ºC for ³2 y. Stable in neutral, moderate alkaline or acidic media. F.p. Forms flammable decomposition products at high temperature
COMMERCIALISATION
History Fungicidal properties of morpholines with large groups attached to the nitrogen described by K. H. König et al. (Angew. Chem., 1965, 77, 327); those of dodemorph by J. Kradel & E. H. Pommer (Proc. Br. Insectic. Fungic. Conf., 4th, 1967, 1, 170). Dodemorph acetate introduced in Germany (1968) by BASF AG. Patents DE 1198125 Manufacturers BASF
APPLICATIONS
dodemorph acetate
Biochemistry Steroid reduction (ergosterol biosynthesis) inhibitor. Mode of action Dodemorph acetate is a systemic fungicide with protective and curative action. Absorbed through the leaves and roots, with translocation. Uses Control of powdery mildews on roses and other ornamentals (outdoors and under glass). Applied at 100 g/100 l (1-2 kg/ha). Phytotoxicity Cinerarias, begonias, and some varieties of rose may be injured. Formulation types EC. Selected products: 'Meltatox' (BASF)
OTHER PRODUCTS
dodemorph acetate
'F238' (BASF); 'Mehltaumittel' (BASF) Discontinued products: 'Milban' * (Grace-Sierra)
ANALYSIS
Product analysis by potentiometric titration with perchloric acid in glacial acetic acid. Residues in crops analysed by glc.
MAMMALIAN TOXICOLOGY
dodemorph
Toxicity class WHO (a.i.) U EC classification Xi; R36/37/38| N; R51, R53
dodemorph acetate
Oral Acute oral LD50 for male rats 3944, female rats 2465 mg/kg. Skin and eye Acute percutaneous LD50 for rats >4000 mg/kg (for 42.6% EC formulation). Skin irritant and severe eye irritant (rabbits). Inhalation LC50 (4 h) for rats 5 mg/l air (for EC formulation). EC classification (Xi; R34, R41| R43| R51, R53)
ECOTOXICOLOGY
dodemorph acetate
Fish LC50 (96 h) for rainbow trout 2.2, guppies c. 40 mg/l. Daphnia LC50 (48 h) 1.8 mg/l. Algae ErC50 (72 h) for Selenastrum capricornutum 1.1 mg/l. Bees Non-toxic to bees; LD50 (oral) >138.8 µg/bee; (contact) >100 µg/bee. Worms LC50 14 d >1000 mg/kg.
ENVIRONMENTAL FATE
Soil/Environment In soil, after 100 d, 23% non-extractable residues, 18% mineralisation and no relevant metabolites were found; DT50 26-73 d. High adsorption, no risk of leaching;Koc 4200-48 000.
|