|
diquat dibromide
Herbicide
HRAC D WSSA 22; bipyridylium
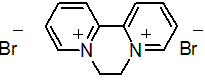
NOMENCLATURE
diquat dibromide
IUPAC name 1,1'-ethylene-2,2'-bipyridyldiylium dibromide
Chemical Abstracts name 6,7-dihydrodipyrido[1,2-a:2',1'-c]pyrazinediium dibromide
CAS RN [85-00-7]; [6385-62-2] for diquat dibromide monohydrate EEC no. 201-579-4
diquat
Common name diquat (BSI, E-ISO, (m) F-ISO, ANSI, WSSA, JMAF); deiquat (Germany); reglon* (former exception, USSR)
IUPAC name 1,1'-ethylene-2,2'-bipyridyldiylium; 9,10-dihydro-8a,10a-diazoniaphenanthrene; 6,7-dihydrodipyrido-[1,2-a:2',1'-c]pyrazine-5,8-di-ium
Chemical Abstracts name 6,7-dihydrodipyrido[1,2-a:2',1'-c]pyrazinediium
CAS RN [2764-72-9] EEC no. 220-433-0
PHYSICAL CHEMISTRY
diquat dibromide
Mol. wt. 344.1 M.f. C12H12Br2N2 Form Colourless to yellow crystals. M.p. decomposes above 325 ºC (monohydrate) V.p. <0.01 mPa (monohydrate) KOW logP = -4.60 (20 ºC) Henry 5 ´ 10-9 Pa m3 mol-1 (calc.) S.g./density 1.61 (25 °C) Solubility In water 700 g/l (20 ºC). Slightly soluble in alcohols and hydroxylic solvents (25 g/l). Insoluble in non-polar organic solvents (<0.1 g/l). Stability Stable in neutral and acidic solutions, but readily hydrolysed in alkaline solutions. DT50 at pH 7 in simulated sunlight c. 74 d. Photochemically decomposed by u.v. irradiation, DT50 <1 w.
diquat
Mol. wt. 184.2 M.f. C12H12N2
COMMERCIALISATION
History Herbicide reported by R. C. Brian et al. (Nature (London), 1958, 181, 446). Introduced by ICI Plant Protection Division (now Syngenta AG) and first marketed in 1962. Patents GB 785732 Manufacturers Syngenta
APPLICATIONS
diquat dibromide
Biochemistry During photosynthesis, superoxide is generated, which damages cell membranes and cytoplasm. Mode of action Non-selective contact herbicide and desiccant, absorbed by the foliage, with some translocation in the xylem. Uses Pre-harvest desiccation of cotton, flax, alfalfa, clover, lupins, oilseed rape, poppies, soya beans, peas, beans, sunflowers, cereals, maize, rice, sugar beet, and other seed crops; destruction of potato haulms; and stripping of hops. Control of annual broad-leaved weeds in vines, pome fruit, stone fruit, bush fruit, strawberries (also control of runners), citrus fruit, olives, hops, vegetables, ornamental plants and shrubs, and other crops. Control of emergent and submerged aquatic weeds. Weed control on non-crop land. Weed control and tassel inhibition in sugar cane. Application rates 400-1000 g/ha. Formulation types SL; Gel. Compatibility Incompatible with alkaline materials, anionic surfactants (e.g. alkyl sulfonates or alkyl aryl sulfonates), and alkali-metal salts of hormone-type herbicides. Selected products: 'Reglone' (Syngenta); mixtures: 'Seccatuttu' (+ paraquat dichloride) (Syngenta)
OTHER PRODUCTS
diquat dibromide
'Boomerang' (GreenCrop); 'Desiquat' (Barclay); 'Midstream' (Scotts UK); 'Reglex' (Isagro) mixtures: 'PDQ' (+ paraquat dichloride) (Syngenta); 'Preeglox L' (+ paraquat dichloride) (Syngenta); 'Allzett' (+ glyphosate-trimesium) (Otsuka); 'Myzet' (+ paraquat dichloride) (Otsuka); 'Priglox' (+ paraquat dichloride) (Nihon Nohyaku); 'Speedway 2' (+ paraquat dichloride) (Scotts UK); 'Spray Seed' (+ paraquat dichloride) (Crop Care) Discontinued products: 'Midstream' * (Zeneca); 'Reglox' * (Zeneca); 'Aquacide' * (Nomix-Chipman) mixtures: 'Parable' * (+ paraquat dichloride) (Zeneca); 'Preglox' * (+ paraquat dichloride) (Zeneca)
ANALYSIS
Product analysis, and mixtures with paraquat, by u.v. spectrophotometry (AOAC Methods, 17th Ed., 969.08; CIPAC Handbook, 1992,E, 74). Free 2,2'-bipyridyl determined by glc (ibid., 1998, H, 153). Residues determined by colorimetry (M. G. Ashley, Pestic. Sci., 1970, 1, 101; A. Calderbank et al., Analyst (London), 1961, 86, 569; A. Calderbank & S. H. Yuen, ibid., 1965, 90, 95; 1966, 91, 625; J. E. Pack, Anal. Methods Pestic., Plant Growth Regul. Food Addit., 1967, 5, 397; J. B. Leary, Anal. Methods Pestic. Plant Growth Regul., 1978, 10, 321; Pestic. Anal. Man., 1979, II). Residues in potatoes by rplc with dual channel u.v. detection (AOAC Methods, 17th Ed., 992.17). Details available from Syngenta.
MAMMALIAN TOXICOLOGY
diquat dibromide
Reviews FAO/WHO 68, 70 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats 408, mice 234 mg/kg. Skin and eye Acute percutaneous LD50 for rats >793 mg/kg. Irritating to skin and eyes (rabbits). Absorption through intact human skin is minimal; exposure can cause irritation and a delay in the healing of cuts and wounds. Can cause temporary damage to nails. Inhalation Extreme exposure to spray droplets may cause nose bleeding. NOEL (2 y) for rats 0.47 mg/kg b.w. daily; (4 y) for dogs 94 mg/kg diet. ADI (JMPR) 0.002 mg cation/kg b.w. [1993]; (EPA) 0.005 mg cation/kg b.w. [1995]. Water GV 10 mg cation/kg b.w. (provisional; based on ADI). Toxicity class WHO (a.i.) II (cation); EPA (formulation) II (a.i.) EC classification T+; R26| T; R48/25| Xn; R22| Xi; R36/37/38| R43| N; R50, R53
ECOTOXICOLOGY
diquat dibromide
Birds Acute oral LD50 for mallard ducks 155, partridges 295 mg/kg. Fish LC50 (96 h) for rainbow trout 39, mirror carp 125 mg/l. Daphnia LC50 (48 h) 2.2 mg/l. Algae EC50 (96 h) 21 mg/l. Bees LD50 (oral, 120 h) 22 mg/bee. Worms LC50 (14 d) 243 mg/kg.
ENVIRONMENTAL FATE
EHC 39 (WHO, 1984). Animals In rats, following oral administration of diquat dibromide, the dose is completely eliminated in the urine and faeces within 4 days. Plants Metabolic breakdown of diquat dibromide does not occur in plants. On plant surfaces, photochemical degradation occurs. Soil/Environment Rapidly degraded by soil micro-organisms, DT50 of unadsorbed diquat <1 w; strong binding in soil increases persistence. Strongly bound and inactivated by soil and aquatic sediments and does not leach into groundwater; Kd >10 000.
|