|
dinotefuran
Insecticide
IRAC 4A; neonicotinoid
NOMENCLATURE
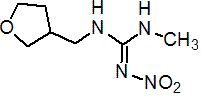
Common name dinotefuran (BSI; pa ISO)
IUPAC name (RS)-1-methyl-2-nitro-3-(tetrahydro-3-furylmethyl)guanidine
Chemical Abstracts name N-methyl-N'-nitro-N''-[(tetrahydro-3-furanyl)methyl]guanidine
CAS RN [165252-70-0] Development codes MTI-446 (Mitsui)
PHYSICAL CHEMISTRY
Mol. wt. 202.2 M.f. C7H14N4O3 M.p. 94.5-101.5 °C KOW logP = -0.644 (pH 7) S.g./density 1.33 Solubility In purified water 54.3?.3 g/l (20 °C). pKa No dissociation in range pH 1.4 to 12.3
COMMERCIALISATION
History Reported by K. Kodaka et al. (Proc. Br. Crop Prot. Conf. - Pests Dis., 1998, 1, 21). Under development by Mitsui Chemicals Inc. Patents EP 0649845 Manufacturers Mitsui
APPLICATIONS
Biochemistry Agonist of the nicotinic acetylcholine receptor, affecting the synapses in the insect central nervous system. Mode of action Active by ingestion and by contact; also exhibits root-systemic activity. Uses Controls a range of hemipterous and other pests, at 100-200 g/ha. Selected products: 'Starkle' (Mitsui)
OTHER PRODUCTS
'Albarin' (Mitsui)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 2804, female rats 2000, male mice 2450, female mice 2275 mg/kg. Skin and eye Acute percutaneous LD50 for male and female rats >2000 mg/kg. Not a skin sensitiser (guinea pigs).
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks 1000, Japanese quail >2000 mg/kg. Fish LC50 (96 h) for carp >1000 ppm; (48 h) for rainbow trout >40 ppm. Daphnia (48 h) 1000 ppm Other aquatic spp. LC50 (48 h) for crayfish 5-10 ppm.
|