|
diflumetorim
Fungicide
FRAC U3, C1; pyrimidinamine
NOMENCLATURE
Common name diflumetorim (BSI, pa ISO)
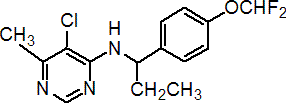
IUPAC name (RS)-5-chloro-N-[1-(4-difluoromethoxyphenyl)propyl]-6-methylpyrimidin-4-ylamine
Chemical Abstracts name (?-5-chloro-N-[1-[4-(difluoromethoxy)phenyl]propyl]-6-methyl-4-pyrimidinamine
CAS RN [130339-07-0] Development codes UBF-002 (Ube)
PHYSICAL CHEMISTRY
Mol. wt. 327.8 M.f. C15H16ClF2N3O Form Pale yellow crystals. M.p. 46.9-48.7 °C V.p. 3.21 ´ 10-1 mPa (25 °C) KOW logP = 4.17 (pH 6.86) Henry 3.19 ´ 10-3 Pa m3 mol-1 (calc.) S.g./density 0.490 (25 °C) Solubility In water 33 mg/l (25 °C). Readily soluble in most organic solvents. Stability Stable to hydrolysis at pH 4-9. pKa 4.5, weak base F.p. 201.3 °C
COMMERCIALISATION
History Invented by Ube Industries, Ltd., and developed in co-operation with Nissan Chemical Industries, Ltd. Registered for ornamental use in Japan in 1997. Acquired by SDS Biotech KK in 2003. Patents US 5141941; EP 0370704 Manufacturers SDS Biotech KK
APPLICATIONS
Biochemistry May inhibit complex 1 of respiration. Mode of action Protectant fungicide. Uses For control of powdery mildew and rust on ornamentals. Formulation types EC. Selected products: 'Pyricut' (SDS Biotech KK, Nissan)
MAMMALIAN TOXICOLOGY
Oral LD50 for male rats 448, female rats 534, male mice 468, female mice 387 mg/kg. Skin and eye Acute percutaneous LD50 for male and female rats >2000 mg/kg. Slight skin and eye irritant (rabbits). Slight skin sensitisation (guinea pigs). Inhalation LC50 for male and female rats 0.61 mg/l. Other Negative in Ames, chromosome aberration and mouse micronucleus tests.
ECOTOXICOLOGY
Birds LD50 for Japanese quail 881, mallard ducks 1979 mg/kg. Fish LC50 (48 h) for rainbow trout 0.025, carp 0.098 mg/l. Daphnia LC50 (3 h) 0.96 mg/l. Bees LD50 (oral) >10 mg/bee; (contact) 29 mg/bee.
ENVIRONMENTAL FATE
Soil/Environment Soil dissipation DT50 (field, Japan) 60-100 d. Aerobic metabolism DT50 4.5 mo; the major metabolite, which barely exceeded 10% at any time, was diflumetorim hydroxylated at the pyrimidine-2 position. Koc 572-1710. Photolytic DT50 (river water) 168 h. See K. Fujii & S. Takamura, Agchem. Japan, 72, 14 (1998).
|