|
diflufenzopyr
Herbicide
HRAC P WSSA 19; semi-carbazone
NOMENCLATURE
Common name diflufenzopyr (BSI, pa ISO)
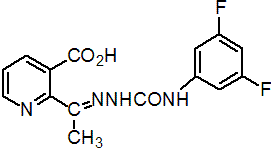
IUPAC name 2-{1-[4-(3,5-difluorophenyl)semicarbazono]ethyl}nicotinic acid
Chemical Abstracts name 2-[1-[[[(3,5-difluorophenyl)amino]carbonyl]hydrazono]ethyl]-3-pyridinecarboxylic acid
CAS RN [109293-97-2]; (sodium salt [109293-98-3]) Development codes BAS 654 00 H; BAS 662 H (mixture with dicamba) (both BASF); SAN 835 H* (acid); SAN 836 H* (sodium salt) (both Sandoz)
PHYSICAL CHEMISTRY
Mol. wt. 334.3 M.f. C15H12F2N4O3 Form Off-white, odourless solid. M.p. 135.5 °C V.p. 1.0 ´ 10-1 mPa (20, 25 °C) KOW logP = 0.037 (pH 7) Henry <7 ´ 10-5 Pa m3 mol-1 (20 °C) S.g./density 0.24 (25 °C) Solubility In water 63 (pH 5), 5850 (pH 7), 10 546 (pH 9) ppm (25 °C). Stability Hydrolysis DT50 13 d (pH 5), 24 d (pH 7), 26 d (pH 9); aqueous photolysis DT50 7 d (pH 5), 17 d (pH 7), 13 d (pH 9). pKa 3.18
COMMERCIALISATION
History Originated by Sandoz Agro (became Novartis Crop Protection AG); development and distribution rights acquired by BASF in December 1996. Mixture with dicamba reported by S. Bowe et al., Proc. Br. Crop Prot. Conf. - Weeds, 1999, 1, 35, and registered in USA and Canada in 1999. Patents US 5098466 Manufacturers BASF
APPLICATIONS
Biochemistry Inhibits auxin transport, apparently by binding with a carrier protein on the plasmalemma. In mixture with dicamba, directs translocation of dicamba to growing points, increasing efficacy of broad-leaved weed control. Tolerance of maize is attributed to rapid metabolism. Mode of action Systemic, post-emergence herbicide. Sensitive broad-leaved plants display epinasty within a few hours; sensitive grasses display stunting. Uses For post-emergence control of annual broad-leaved and perennial weeds in maize, pasture/rangeland, and non-crop areas. Initial commercialisation of a mixture with dicamba, both materials as sodium salts. Applied at 0.05-0.1 lb diflufenzopyr/a. Formulation types WG. Selected products: mixtures: 'Distinct' (+ dicamba-sodium) (diflufenzopyr also as sodium salt) (BASF); 'Overdrive' (+ dicamba-sodium) (diflufenzopyr also as sodium salt) (BASF)
OTHER PRODUCTS
Mixtures: 'Celebrity Plus' (+ nicosulfuron+ dicamba-sodium) (diflufenzopyr also as sodium salt) (BASF)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male and female rats >5.0 g/kg. Skin and eye Acute percutaneous LD50 for male and female rabbits >5.0 g/kg. Mild eye irritant; not a skin irritant (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 for rats >2.93 mg/l. NOEL NOAEL (1 y) for dogs 750 ppm (26 mg/kg b.w. daily for males, 28 mg/kg b.w. daily for females). ADI 0.26 mg/kg. Other Non-teratogenic; non-carcinogenic. Toxicity class EPA (formulation) III ('Distinct')
ECOTOXICOLOGY
Birds LD50 for bobwhite quail >2250 mg/kg. LC50 for bobwhite quail and mallard ducks >5620 ppm. Fish LC50 (96 h) for bluegill sunfish >135 ppm, rainbow trout 106 mg/l. Daphnia EC50 (48 h) 15 mg/l. Algae EC50 (5 d) for Selenastrum 0.11 mg/l. Bees LD50 (contact) >90 mg/bee.
ENVIRONMENTAL FATE
Animals Following oral administration, diflufenzopyr was partially absorbed and rapidly eliminated; 20-44 % of the dose was eliminated in urine and 49-79 % in faeces. By contrast, intravenously dosed rats excreted 61-89% of the dose in urine. Elimination DT50 in urine and faeces was c. 6 h. Total radioactive residues in tissues <3% of the administered dose. Diflufenzopyr was eliminated primarily as unchanged parent compound. Diflufenzopyr was also rapidly eliminated, largely unchanged, for hens and goats. Soil/Environment DT50 for photolysis on soil 14 d, for aerobic soil metabolism (lab.) 8-10 d, for aerobic aquatic metabolism 20-26 d. Average DT50 in field soil 4.5 d. Very mobile (Koc 18-156 ml/g); metabolites also very mobile. However, based upon proposed use, US EPA does not expect diflufenzopyr to reach drinking water.
|