|
diflufenican
Herbicide
HRAC F1 WSSA 12; pyridinecarboxamide
NOMENCLATURE
Common name diflufenican (BSI, draft E-ISO, (m) draft F-ISO); diflufenicanil ((m) France)
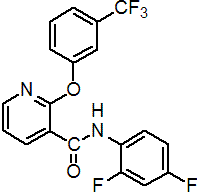
IUPAC name 2',4'-difluoro-2-(a,a,a-trifluoro-m-tolyloxy)nicotinanilide
Chemical Abstracts name N-(2,4-difluorophenyl)-2-[3-(trifluoromethyl)phenoxy]-3-pyridinecarboxamide
Other names DFF CAS RN [83164-33-4] Development codes M&B 38 544 (May & Baker)
PHYSICAL CHEMISTRY
Composition ³97% pure. Mol. wt. 394.3 M.f. C19H11F5N2O2 Form Colourless crystals. M.p. 159-161 °C V.p. 4.25 ´ 10-3 mPa (25 °C, gas saturation method) KOW logP = 4.9 Henry 0.033 Pa m3 mol-1 Solubility In water <0.05 mg/l (25 ºC). Soluble in most organic solvents, e.g. acetone, dimethylformamide 100, acetophenone, cyclohexanone 50, isophorone 35, xylene 20, cyclohexane, 2-ethoxyethanol, kerosene <10 (all in g/kg, 20 ºC). Stability Stable in air up to melting point. At 22 ºC, very stable in aqueous solution at pH 5, 7 and 9. Fairly stable to photolysis.
COMMERCIALISATION
History Herbicide reported by M. C. C. Ramp et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1985, 1, 23) and by C. F. A. Kyndt et al. (ibid., p. 29). Introduced by May & Baker Ltd (now Bayer CropScience). Patents EP 53011 Manufacturers Bayer CropScience; Shenyang
APPLICATIONS
Biochemistry Blocks carotenoid biosynthesis, by inhibition of phytoene desaturase. Mode of action Selective contact and residual herbicide, absorbed principally by the shoots of germinating seedlings, with limited translocation. Uses Applied at 125-250 g/ha pre- or early post-emergence in autumn-sown wheat and barley, to control grass and broad-leaved weeds, particularly Galium, Veronica and Viola spp. Normally used in combination with isoproturon or other cereal herbicides. Phytotoxicity Any slight phytotoxicity is in the form of small transient patches on basal leaves, with no adverse effect on crop development. Formulation types SC; WP. Selected products: 'Fenican' (Bayer CropScience); 'Legacy' (Makhteshim-Agan); 'Tigrex' (Bayer CropScience); mixtures: 'Bacara' (+ flurtamone) (Bayer CropScience); 'Javelin' (+ isoproturon) (Bayer CropScience)
OTHER PRODUCTS
'Blizzard' (Bayer CropScience); 'Brodal' (Bayer CropScience); 'Econal' (Bayer CropScience); 'Ioniz' (Bayer CropScience); 'Legato' (Makhteshim-Agan); 'Zodiac TX' (Bayer CropScience) mixtures: 'Amazon' (+ clodinafop-propargyl) (Bayer CropScience, Syngenta); 'Ardent' (+ trifluralin) (Bayer CropScience); 'Azur' (+ ioxynil+ isoproturon) (as salts) (Bayer CropScience); 'Bolero' (+ terbuthylazine) (Syngenta); 'Capture' (+ ioxynil+ bromoxynil) (Bayer CropScience); 'Carat' (+ flurtamone) (Bayer CropScience); 'Chamois' (+ ioxynil+ bromoxynil) (Philagro); 'Cougar' (+ isoproturon) (Bayer CropScience); 'DFF 625' (+ isoproturon) (Landgold); 'Dièze' (+ mecoprop-P+ bromoxynil) (Bayer CropScience); 'Etnos' (+ isoproturon+ pyraflufen-ethyl) (Bayer CropScience); 'Fenikan' (+ isoproturon) (Bayer CropScience); 'First' (+ ioxynil+ bromoxynil) (Bayer CropScience); 'Galace' (+ trifluralin) (Dow AgroSciences); 'Graduate' (+ flurtamone) (Bayer CropScience); 'Grenadier' (+ isoproturon) (Bayer CropScience); 'Halbard' (+ cyanazine) (Feinchemie Schwebda); 'Helmsman' (+ oxadiazon+ carbetamide) (Bayer CropScience); 'Herold' (+ flufenacet) (Bayer CropScience); 'Ingot' (+ flurtamone+ isoproturon) (Bayer CropScience); 'Javelin Gold' (+ isoproturon) (Bayer CropScience); 'Legato Plus' (+ isoproturon) (Makhteshim-Agan); 'Lucifer' (+ clodinafop-propargyl) (Bayer CropScience); 'Panther' (+ isoproturon) (Bayer CropScience); 'Quartz' (+ isoproturon) (Bayer CropScience); 'Snap!' (+ flurtamone) (Me2); 'Spearhead' (+ MCPA+ clopyralid) (Bayer CropScience); 'Traviata' (+ mecoprop-P+ bromoxynil) (Bayer CropScience) Discontinued products mixtures: 'Tolkan Turbo' * (+ isoproturon) (Aventis); 'Amazon TP' * (+ clodinafop-propargyl) (Novartis)
ANALYSIS
Product analysis by rplc (CIPAC Handbook, 1998, H, 147). Methods have been developed to determine residues in plants and soil (J. Agric. Food Chem. 1991, 39(5), 968-76).
MAMMALIAN TOXICOLOGY
Reviews Proc. Br. Crop Prot. Conf. - Weeds, 1985, 1, 23-28. Oral Acute oral LD50 for rats >2000, mice >1000, rabbits >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin and eyes (rabbits). Inhalation LC50 (4 h) for rats >2.34 mg/l air. NOEL In 14 d sub-acute trials in rats, no adverse effect observed at 1600 mg/kg b.w. In 90 d feeding trials, NOEL for dogs was 1000 mg/kg b.w. daily, for rats 500 ppm diet. Other Non-mutagenic in the Ames test. Toxicity class WHO (a.i.) U EC classification R52, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for quail >2150, mallard ducks >4000 mg/kg. Fish LC50 (96 h) for rainbow trout 56-100, carp 105 mg/l. Daphnia LC50 (48 h) - no effect at 10 mg/l. Algae No growth inhibition of algae (96 h) at 10 mg/l. Bees Non-toxic by ingestion or contact. Worms Non-toxic to earthworms.
ENVIRONMENTAL FATE
Plants In cereals, rapidly metabolised via the nicotinamide and nicotinic acid to CO2. Following pre-emergence application in autumn, no residues are detectable in the grain and straw after c. 200-250 days. Soil/Environment In soil, degradation proceeds via the metabolites 2-(3-trifluoromethylphenoxy)nicotinamide and 2-(3-trifluoromethylphenoxy)nicotinic acid to bound residues and CO2. Half-life varies from 15 to 30 weeks, depending on soil type and water content.
|