|
dazomet
Nematicide, fungicide, herbicide, insecticide
HRAC Z
NOMENCLATURE
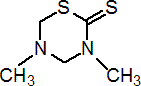
Common name dazomet (BSI, E-ISO, (m) F-ISO, WSSA, JMAF); tiazon (USSR); DMTT* (former WSSA name)
IUPAC name 3,5-dimethyl-1,3,5-thiadiazinane-2-thione; tetrahydro-3,5-dimethyl-1,3,5-thiadiazine-2-thione
Chemical Abstracts name tetrahydro-3,5-dimethyl-2H-1,3,5-thiadiazine-2-thione
CAS RN [533-74-4] EEC no. 208-576-7 Development codes N-521 (Stauffer); Crag Fungicide 974 (Rhône-Poulenc); BAS 002 01N (BASF)
PHYSICAL CHEMISTRY
Composition ³94% pure. Mol. wt. 162.3 M.f. C5H10N2S2 Form Colourless crystals. M.p. 104-105 ºC (decomp.; tech. grade) V.p. 0.37 mPa (20 ºC) KOW logP = 0.63 (pH 7) Henry 2.69 ´ 10-5 Pa m3 mol-1 S.g./density 1.37 (tech.) Solubility In water 3 g/kg (20 ºC). In cyclohexane 400, chloroform 391, acetone 173, benzene 51, ethanol 15, diethyl ether 6 (all in g/kg, 20 ºC). Stability Stable at temperatures up to 35 ºC. Sensitive to temperatures >50 ºC, and to moisture.
COMMERCIALISATION
History Originally prepared by M. Delépine (Bull. Soc. Chim. Fr., 1897, 15, 891), later introduced as a soil fumigant. Manufacturers BASF
APPLICATIONS
Biochemistry Non-selective inhibition of enzymes by degradation products. Mode of action A pre-planting soil fumigant, acting by decomposition to methyl isothiocyanate. Uses Soil sterilant, applied prior to planting out crops, at 200-600 kg/ha. Controls soil fungi (Fusarium, Pythium, Rhizoctonia, Sclerotinia and Verticillium spp. and Colletotrichum coccodes), nematodes, germinating weed seeds, bacteria and soil insects. Also used as a slimicide in pulp and paper manufacture, and as a preservative in adhesives and glues. Phytotoxicity Highly phytotoxic to all green plants. Treated soils should not be planted until shown to be free of dazomet and its degradation products. Formulation types GR. Selected products: 'Basamid' (BASF); 'Dacron' (Vapco)
OTHER PRODUCTS
'Dazoberg' (Diachem) Discontinued products: 'Mylone' * (Union Carbide)
ANALYSIS
Product determination of the active ingredient by HPLC (CIPAC Handbook, J, 37 (2000), method 146/TC/M). Residues of parent compound in soil by hplc; of methyl isothiocyanate in crops by glc. Details available from BASF.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 520, male mice 455, female mice 710, rabbits 320-620 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg; DP formulation is a skin and eye irritant to rabbits. Inhalation LC50 (4 h) for rats 8.4 mg/l air. NOEL in rats 20 mg/kg diet. ADI 0.009 mg/kg b.w. (BASF proposed) Other Not oncogenic. Toxicity class WHO (a.i.) III; EPA (formulation) III EC classification Xn; R22| Xi; R36| N; R50, R53
ECOTOXICOLOGY
Birds LD50 for bobwhite quail 415 mg/kg b.w. LC50 for bobwhite quail 1850, mallard ducks >5000 mg/kg diet. Fish LC50 (96 h) 0.16 mg/l. Daphnia EC50 (48 h) 0.3 mg/l. Algae EC50 (96 h) for Scenedesmus subspicatus 1.0 mg/l. Other aquatic spp. EC10 (17 h) for Pseudomonas putida 1.8 mg/l. Bees Not toxic to bees when used as directed. Worms Toxic to worms (used as a soil sterilant).
ENVIRONMENTAL FATE
Plants Following application to strawberries, no residues of dazomet or of its degradation products methyl isothiocyanate, dimethyl- or monomethylthiourea were detected at >0.01 ppm in the fruit. Soil/Environment In soil DT50 <1 d, in water DT50 <10 h (pH >5). In soil, in the presence of moisture, degrades to methyl isocyanate, formaldehyde, hydrogen sulfide and methylamine.
|