|
cymoxanil
Fungicide
FRAC 27; cyanoacetamide oxime
NOMENCLATURE
Common name cymoxanil (BSI, ANSI, draft E-ISO, (m) draft F-ISO)
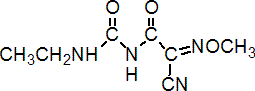
IUPAC name 1-(2-cyano-2-methoxyiminoacetyl)-3-ethylurea
Chemical Abstracts name 2-cyano-N-[(ethylamino)carbonyl]-2-(methoxyimino)acetamide
CAS RN [57966-95-7] EEC no. 261-043-0 Development codes DPX-T3217 (DuPont)
PHYSICAL CHEMISTRY
Composition Tech. is >97%. Mol. wt. 198.2 M.f. C7H10N4O3 Form Colourless, odourless crystals; (tech. is a peach colour). M.p. 160-161 ºC; (tech., 159-160 °C) V.p. 0.15 mPa (20 °C) KOW logP = 0.59 (pH 5), 0.67 (pH 7) Henry 3.8 ´ 10-5 (pH 7), 3.3 ´ 10-5 (pH 5) (both Pa m3 mol-1, calc.) S.g./density 1.32 (25 ºC) Solubility In water 890 mg/kg (pH 5, 20 ºC). In hexane 0.037, toluene 5.29, acetonitrile 57, ethyl acetate 28, n-octanol 1.43, methanol 22.9, acetone 62.4, dichloromethane 133.0 (all in g/l, 20 ºC). Stability Hydrolysis DT50 148 d (pH 5), 34 h (pH 7), 31 min (pH 9). Aqueous photolysis DT50 1.8 d (pH 5). pKa 9.7 (decomp.)
COMMERCIALISATION
History Fungicide reported by J. M. Serres & G. A. Carraro (Meded. Fac. Landbouwwet. Rijksuniv. Gent, 1976. 41, 645). Introduced by E. I. du Pont de Nemours Co.; first marketed in mid 1970s. Patents US 3957847 Manufacturers DuPont; Griffin; Oxon; Sannong; Sharda
APPLICATIONS
Mode of action Foliar fungicide with protective and curative action. Has contact and local systemic activity, and also inhibits sporulation. Uses Control of Peronosporales, especially Peronospora, Phytophthora, and Plasmopara spp. Normally used in combination with protectant fungicides (to improve residual activity) on a range of crops, including vines, hops, potatoes, and tomatoes. Formulation types WP; WG. Compatibility Incompatible with alkaline materials. Selected products: 'Curzate' (DuPont); 'Asco' (Agrimix); 'Texas' (Rocca); mixtures: 'Equation Pro' (+ famoxadone) (DuPont); 'Tanos' (+ famoxadone) (DuPont); 'Aktuan' (+ dithianon) (BASF, Stähler); 'Aviso' (+ metiram) (BASF); 'Curtine-V' (+ mancozeb) (Vapco); 'Duett' (+ mancozeb) (Cequisa); 'Fobeci' (+ folpet+ benalaxyl) (Sipcam Inagra); 'Glover' (+ copper sulfate) (Cequisa); 'Manex C-8' (+ mancozeb) (Griffin); 'Micexanil' (+ mancozeb) (Sipcam); 'Pulsan' (+ mancozeb+ oxadixyl) (Syngenta); 'Quadris' (+ azoxystrobin) (Syngenta); 'Sygan PM' (+ folpet+ mancozeb) (Griffin); 'Syphal PM' (+ folpet+ mancozeb+ copper oxychloride) (Griffin)
OTHER PRODUCTS
'Cimoxpron' (Probelte); 'Sarman' (Sipcam Phyteurop); 'Vitene' (Sipcam); 'Zetanil' (Sipcam) mixtures: 'Besiege WSB' (+ mancozeb) (DuPont); 'Copral' (+ copper sulfate) (DuPont); 'Curzate M68' (+ mancozeb) (DuPont); 'Horizon' (+ famoxadone) (DuPont, Nissan); 'Iteral' (+ famoxadone) (DuPont); 'Sirdate S' (+ folpet+ oxadixyl) (DuPont); 'Sygan LS' (+ folpet+ mancozeb) (DuPont); 'Sygan S' (+ folpet) (DuPont); 'Syphal LS' (+ folpet+ mancozeb+ copper oxychloride) (DuPont); 'Systol M' (+ mancozeb) (DuPont); 'Trustan' (+ mancozeb+ oxadixyl) (DuPont); 'Apron Elite' (+ oxadixyl+ thiram+ carbendazim) (Syngenta); 'Aviso Cup' (+ metiram+ copper oxychloride) (BASF); 'Blizzard' (+ chlorothalonil) (Nihon Nohyaku); 'Control III' (+ copper hydroxide) (Ingeniería Industrial); 'Cupertine Super' (+ Bordeaux mixture) (IQV); 'Curathane' (+ mancozeb) (Dow AgroSciences); 'Cymoxeb' (+ mancozeb) (Me2); 'Eclair' (+ trifloxystrobin) (Bayer CropScience); 'Harpon' (+ zoxamide) (Dow AgroSciences); 'Kupfer Fusilan' (+ copper oxychloride) (Kwizda); 'Manoxil' (+ mancozeb) (AgroSan); 'Rhythm' (+ mancozeb) (Interfarm); 'Ripost' (+ mancozeb+ oxadixyl) (Syngenta); 'Sirdate P' (+ maneb+ oxadixyl) (Griffin); 'Tertio' (+ fenamidone+ fosetyl-aluminium) (Bayer CropScience); 'Valiant' (+ folpet+ fosetyl-aluminium) (Bayer CropScience); 'Vironex' (+ folpet) (IQV); 'Wakil XL' (+ fludioxonil+ metalaxyl-M) (Syngenta); 'Wakil' (+ oxadixyl+ thiram+ carbendazim) (Syngenta) Discontinued products mixtures: 'Besiege' * (+ mancozeb) (DuPont); 'Ashlade Solace' * (+ mancozeb) (Nufarm Whyte, Ashlade); 'Cymotine' * (+ mancozeb) (Agro Chemicals); 'Fytospore' * (+ mancozeb) (Zeneca)
ANALYSIS
Product analysis by hplc. Residues determined by glc. Details available from DuPont.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 760, female rats 1200 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Not an eye irritant; slight skin irritant (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for male and female rats >5.06 mg/l. NOEL (2 y) for male rats 4.1, female rats 5.4, male mice 4.2, female mice 5.8, male and female dogs 3.0 mg/kg b.w. daily. ADI 0.013 mg/kg. Toxicity class WHO (a.i.) III; EPA (formulation) III EC classification Xn; R22| R43| N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail and mallard ducks >2250 mg/kg. Dietary LC50 (8 d) for bobwhite quail and mallard ducks >5620 mg/kg diet. Fish LC50 (96 h) for rainbow trout 61, bluegill sunfish 29, common carp 91, sheepshead minnow >47.5 mg/l. Daphnia LC50 (48 h) 27 mg/l. Algae EC50 (5 d) for Selenastrum capricornutum 1.21 mg/l; EC50 for Anabaena flos-aquae 231 ppb. Other aquatic spp. LC50 (96 h) for Eastern oyster >46.9, mysid shrimp >44.4 ppm. Bees Not toxic to bees; LD50 (48 h, contact) >25 mg/bee; LC50 (48 h, oral) >1000 ppm. Worms LC50 (14 d) >2208 mg/kg soil.
ENVIRONMENTAL FATE
Animals In rats, cymoxanil was readily absorbed through the intestines; the majority of the dose was excreted in the urine. The proposed metabolic pathway involves hydrolysis of cymoxanil and then degradation to glycine. Plants Degraded to glycine with subsequent incorporation into natural products (proteins and starch). Soil/Environment In lab. soils, DT50 0.75-1.6 d (5 soils, pH range 5.7-7.8, o.m. 0.8-3.5%). In the field, DT50 (bare soil) 0.9-9 d. In aquatic studies, DT50 <1 d. Koc 38-237. Cymoxanil is mobile, adsorption Freundlich K 0.29 to 2.86 in four soil types.
|