|
cyhalothrin
Insecticide
IRAC 3; pyrethroid
NOMENCLATURE
Common name cyhalothrin (BSI, draft E-ISO, BAN); cyhalothrine ((f) draft F-ISO)
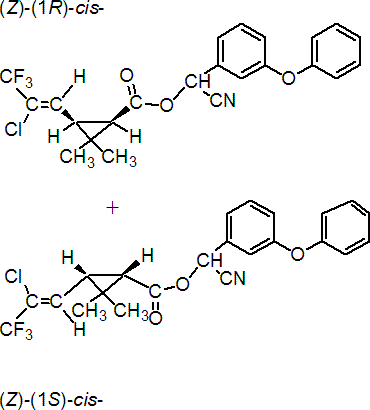
IUPAC name (RS)-a-cyano-3-phenoxybenzyl (Z)-(1RS,3RS)-3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclopropanecarboxylate
Roth: (RS)-a-cyano-3-phenoxybenzyl (Z)-(1RS)-cis-3-(2-chloro-3,3,3-trifluoropropenyl)-2,2-dimethylcyclopropanecarboxylate
Chemical Abstracts name [1a,3a(Z)]-(?-cyano-(3-phenoxyphenyl)methyl 3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylate
CAS RN [68085-85-8] EEC no. 268-450-2 Development codes PP563; ICI 146 814 (both ICI) Official codes OMS 2011
PHYSICAL CHEMISTRY
Composition Tech. grade cyhalothrin has purity ³90% m/m, of which ³95% is cis- isomers. Mol. wt. 449.9 M.f. C23H19ClF3NO3 Form Yellow to brown viscous oil (tech.). B.p. Does not boil at atmospheric pressure V.p. 0.001 mPa (20 ºC, est.) KOW logP = 6.9 (20 ºC) S.g./density 1.25 (25 ºC) Solubility In water 0.005 mg/l (pH 6.5, 20 ºC). In acetone, dichloromethane, methanol, diethyl ether, ethyl acetate, hexane, toluene, all >500 g/l (20 ºC). Stability Stable to decomposition and cis/trans isomerisation for at least 4 years in the dark at 50 ºC. Stable to light; loss on storage in the light is <10% in 20 months. Decomposes at 275 ºC. Slowly hydrolysed by water in sunlight at pH 7-9, more rapidly at pH >9. pKa >9 (hydrolysis prevents measurement) F.p. 204 °C (tech., Pensky-Martens closed cup)
COMMERCIALISATION
History Insecticide reported by P. D. Bentley et al. (Pestic. Sci., 1980, 11, 156) and its acaricidal properties by V. K. Stubbs (Austral. Vet. J., 1982, 59, 152). Introduced by ICI Australia (now Crop Care Australasia Pty Ltd) and ICI Agrochemicals (now Syngenta AG) in the 1980s. Patents AU 521136; US 4183948; GB 2000764 Manufacturers Syngenta
APPLICATIONS
Biochemistry Acts on the nervous system of insects, disturbs the function of neurons by interaction with the sodium channel. Mode of action Non-systemic insecticide with contact and stomach action, and repellent properties. Gives rapid knockdown and long residual activity. Uses Control of animal ectoparasites, especially Boophilus microplus and Haematobia irritans on cattle, and Bovicola ovis, Linognathus spp. and Melophagus ovinus on sheep. Applied as an animal dip or as a spray around animal houses. Formulation types EC; WP. Selected products: 'Cyhalon' (Syngenta); 'Grenade' (Syngenta)
ANALYSIS
Product analysis of pyrethroids reviewed by E. Papadopoulou-Mourkidou in Comp. Analyt. Profiles. By hplc (A. Jacques et al., Anal. Methods Pestic. Plant Growth Regul., 1984, 13, 9). Residues determined by glc with ECD (idem, ibid.). Details available from Syngenta.
MAMMALIAN TOXICOLOGY
Reviews JECFA 54, FAO/WHO 42, 43 (see part 2 of the Bibliography). Oral Acute oral LD50 for male rats 166, female rats 114, guinea pigs >5000, rabbits >1000 mg/kg. Skin and eye Acute percutaneous LD50 for male rats 1000-2500, female rats 200-2500, rabbits >2500 mg/kg. Moderate eye irritant; not a skin irritant (rabbits). Moderate skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >0.086 mg/l. NOEL No significant toxicological effect observed in feeding trials with rats (2 y) or dogs (0.5 y) at 2.5 mg/kg daily. ADI (JECFA) 0.002 mg/kg b.w. (temporary) [2000]; (JMPR) 0.02 mg/kg b.w. [1984]. Other No evidence of carcinogenicity, mutagenicity or disturbed reproductive functions; no adverse effects on foetal development. A facial sensation may be experienced by users of the chemical; this is transient, with complete recovery. Toxicity class WHO (a.i.) II
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >5000 mg/kg. Fish LC50 (96 h) for rainbow trout 0.00054 mg/l. Daphnia LC50 (48 h) 0.38 mg/l.
ENVIRONMENTAL FATE
EHC 99 (WHO, 1990). Animals In rats, following oral administration, cyhalothrin is rapidly eliminated in urine and faeces. The ester group is hydrolysed, both moieties forming polar conjugates. Soil/Environment In soil, DT50 c. 4-12 weeks. Leaching of cyhalothrin and its degradation products through a range of soil types is negligible.
|