|
cyfluthrin
Insecticide
IRAC 3; pyrethroid
NOMENCLATURE
Common name cyfluthrin (BSI, draft E-ISO, BAN); cyfluthrine ((f) draft F-ISO)
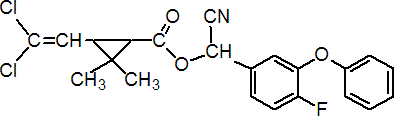
IUPAC name (RS)-a-cyano-4-fluoro-3-phenoxybenzyl (1RS,3RS;1RS,3SR)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
Roth: (RS)-a-cyano-4-fluoro-3-phenoxybenzyl (1RS)-cis-trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
Chemical Abstracts name cyano(4-fluoro-3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate (unstated stereochemistry)
CAS RN [68359-37-5] unstated stereochemistry EEC no. 269-855-7 Development codes BAY FCR 1272 (Bayer) Official codes OMS 2012
PHYSICAL CHEMISTRY
Composition Comprises a mixture of four diastereoisomeric pairs of enantiomers:
I (R)-a-cyano-4-fluoro-3-phenoxybenzyl (1R)-cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate + (S)-a, (1S)-cis-;
II (S)-a, (1R)-cis- + (R)-a, (1S)-cis-;
III (R)-a, (1R)-trans- + (S)-a, (1S)-trans-;
IV (S)-a, (1R)-trans- + (R)-a, (1S)-trans- .
Tech. grade contains: 23-27% diastereoisomer I, 17-21% diastereoisomer II, 32-36% diastereoisomer III, and 21-25% diastereoisomer IV. Mol. wt. 434.3 M.f. C22H18Cl2FNO3 Form Colourless crystals; (tech. is a brown, oily, viscous mass, with crystalline parts). M.p. (I) 64 ºC; (II) 81 ºC; (III) 65 ºC; (IV) 106 ºC; tech., c. 60 ºC B.p. decomp. >220 °C V.p. (I) 9.6 ´ 10-4; (II) 1.4 ´ 10-5; (III) 2.1 ´ 10-5; (IV) 8.5 ´ 10-5 (all in mPa, 20 ºC) KOW logP: (I) 6.0; (II) 5.9; (III) 6.0; (IV) 5.9 (all 20 °C) Henry (I) 1.9 ´ 10-1; (II) 2.9 ´ 10-3; (III) 4.2 ´ 10-3; (IV) 1.3 ´ 10-2 (all in Pa m3 mol-1, 20 °C) S.g./density 1.28 (20 ºC) Solubility Diastereoisomer I: In water 2.5 (pH 3), 2.2 (pH 7) (both in mg/l, 20 ºC). In dichloromethane, toluene >200, n-hexane 10-20, isopropanol 20-50 (all in g/l, 20 ºC). Diastereoisomer II: In water 2.1 (pH 3), 1.9 (pH 7) (both in mg/l, 20 ºC). In dichloromethane, toluene >200, n-hexane 10-20, isopropanol 5-10 (all in g/l, 20 ºC). Diastereoisomer III: In water 3.2 (pH 3), 2.2 (pH 7) (both in mg/l, 20 ºC). In dichloromethane, toluene >200, n-hexane, isopropanol 10-20 (all in g/l, 20 ºC). Diastereoisomer IV: In water 4.3 (pH 3), 2.9 (pH 7) (both in mg/l, 20 ºC). In dichloromethane >200, toluene 100-200, n-hexane 1-2, isopropanol 2-5 (all in g/l, 20 ºC). Stability Thermally stable at room temperature. In water, DT50 for diastereoisomer I: 36, 17, 7; II 117, 20, 6; III 30, 11, 3; IV 25, 11, 5 (all in days, pH 4, 7, 9 respectively, 22 ºC). F.p. 107 ºC (tech.)
COMMERCIALISATION
History Insecticide reported by I. Hammann & R. Fuchs (Pflanzenschutz-Nachr. (Engl. Ed.), 1981, 34, 121) and W. Behrenz et al. (ibid., 1983, 36, 127). Introduced by Bayer AG in 1983. Patents DE 2709264 Manufacturers Bayer CropScience; Jiangsu Yangnong; Mitchell Cotts; Sundat
APPLICATIONS
Biochemistry Acts on the nervous system of insects, disturbs the function of neurons by interaction with the sodium channel. Mode of action Non-systemic insecticide with contact and stomach action. Acts on the nervous system, with rapid knockdown and long residual activity. Uses An insecticide effective against many pests, especially Lepidoptera, Coleoptera, Homoptera and Hemiptera on cereals, cotton, fruit and vegetables; also against migratory locusts and grasshoppers. For agricultural uses, applied at 15-40 g/ha. Used against Blattellidae, Culicidae and Muscidae in public health situations, stored products, domestic use and animal health. It has a rapid knockdown effect and long-lasting residual activity. Formulation types AE; EC; EO; ES; EW; GR; UL; WP. Compatibility Incompatible with azocyclotin. Selected products: 'Baygon aerosol' (public health) (Makhteshim-Agan, Bayer CropScience); 'Bayofly' (Makhteshim-Agan, Bayer CropScience); 'Baythroid' (agricultural use) (Makhteshim-Agan, Bayer CropScience); 'Hidalgroc' (Rocca); mixtures: 'Leverage' (+ imidacloprid) (USA) (Makhteshim-Agan, Bayer CropScience); 'Aztec' (+ tebupirimfos) (Amvac, Bayer CropScience, Makhteshim-Agan)
OTHER PRODUCTS
'Tempo' (Makhteshim-Agan, Bayer CropScience) mixtures: 'Confidor Supra' (+ imidacloprid) (Makhteshim-Agan, Bayer CropScience); 'Legend' (+ imidacloprid) (Makhteshim-Agan, Bayer CropScience); 'Appeal' (+ codlemone) (Bayer CropScience); 'Bayflor Duo' (+ bitertanol) (Bayer CropScience, Makhteshim-Agan)
ANALYSIS
Product analysis, and diastereoisomer ratio determined by hplc with u.v. detection (CIPAC Handbook, 1998, H, 106). Residues determined by glc (Anal. Methods Residues Pestic., 1988, Part I, M11). Details of methods for product and residue analysis available from Bayer CropScience. Analysis of pyrethroids reviewed by E. Papadopoulou-Mourkidou in Comp. Analyt. Profiles.
MAMMALIAN TOXICOLOGY
Reviews JECFA 48; FAO/WHO 50, 52 (see part 2 of Bibliography). Oral Acute oral LD50 for rats c. 500 mg/kg (in xylol), c. 900 mg/kg (PEG 400), c. 20 mg/kg (water/cremophor); for dogs >100 mg/kg. Skin and eye Acute percutaneous LD50 (24 h) for male and female rats >5000 mg/kg. Non-irritating to skin; mildly irritating to eyes (rabbits). Inhalation LC50 (4 h) for male and female rats 0.5 mg/l air (aerosol). NOEL (2 y) for rats 50, mice 200 mg/kg diet; (1 y) for dogs 160 mg/kg diet. ADI 0.02 mg/kg b.w. [1997] (JECFA evaluation); (JMPR) 0.02 mg/kg b.w. [1987]. Toxicity class WHO (a.i.) II; EPA (formulation) II EC classification T+; R28| T; R23| N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Fish LC50 (96 h) for golden orfe 0.0032, rainbow trout 0.00047, bluegill sunfish 0.0015 mg/l. Daphnia LC50 (48 h) 0.00016 mg/l. Algae ErC50 for Scenedesmus subspicatus >10 mg/l. Bees Toxic to honeybees. Worms LC50 for Eisenia foetida >1000 mg/kg dry soil.
ENVIRONMENTAL FATE
Animals Cyfluthrin was largely and very quickly eliminated; 97% of the administered amount was eliminated after 48 h via the urine and the faeces. Plants Since cyfluthrin is not systemic, it does not penetrate into the plant tissues and does not translocate into other parts of the plant. Soil/Environment Degradation in different soils is rapid. The leaching behaviour can be classified as immobile. The metabolites of cyfluthrin are subject to further microbial degradation to the point of mineralisation to CO2.
|