|
cuprous oxide
Fungicide
FRAC M1; multi-site: inorganic
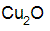
NOMENCLATURE
Common name cuprous oxide (E-ISO, accepted in lieu of common name); oxyde cuivreux (F-ISO, accepted in lieu of common name)
IUPAC name copper(I) oxide; dicopper oxide
Chemical Abstracts name copper oxide (Cu2O)
CAS RN [1317-39-1] EEC no. 215-270-7
PHYSICAL CHEMISTRY
Composition Contains 86% Cu++. Mol. wt. 143.1 M.f. Cu2O Form Red-brown powder. M.p. 1235 ºC B.p. 1800 ºC V.p. Negligible Solubility Practically insoluble in water and organic solvents. Soluble in dilute mineral acids and in aqueous ammonia and its salts. Stability Liable to oxidation to cupric oxide and to conversion to a carbonate on exposure to moist air.
COMMERCIALISATION
History Fungicidal properties as seed protectant reported by J. G. Horsfall (N. Y. St. Agric. Exp. Stn. Bull., 1932, No. 615). First marketed by Sandoz AG in 1943. Subsequently used for foliage protection. Manufacturers Ingeniería Industrial; Nordox; Sulcosa; Syngenta
APPLICATIONS
Biochemistry Copper-II ion (Cu++) is taken up by the spores during germination and accumulates until a sufficiently high concentration is achieved to kill the spore cell; the activity is limited to the prevention of spore germination. Mode of action Foliar fungicide with preventative action. Deposits must be on the crop before fungal spores begin to germinate. Uses Control of blights, downy mildews, rusts, and leaf spot diseases in a wide range of crops, including potatoes, tomatoes, vines, hops, olives, pome fruit, stone fruit, citrus fruit, beetroot, sugar beet, celery, carrots, coffee, cocoa, tea, bananas, etc. Applied at 0.75-1 kg/ha. Phytotoxicity Non-phytotoxic when used as directed, except to brassicas and copper-sensitive plants. Russetting is possible with some varieties of fruit. Formulation types WG; WP. Compatibility Not compatible with highly alkaline pesticides, or with fenvalerate, parathion, chlorpyrifos or dicloran. Selected products: 'Copper Nordox' (Nordox); 'Copper-Sandoz' (Syngenta); 'Cúprox' (Ingeniería Industrial)
OTHER PRODUCTS
'Chem Copp' (Chemet) Discontinued products: 'Yellow Cuprocide' * (Rohm & Haas)
ANALYSIS
Product determined iodometrically or by conversion to sulfate followed by electrolytic determination (CIPAC Handbook, 1992, E,42; ibid., 1998, H, 96; AOAC Methods, 1984, 6.015-6.016; MAFF Ref. Book, 1958, No. 1, p. 16); metallic copper in cuprous oxide may be determined (L. C. Hurd & A. R. Clark, Ind. Eng. Chem. Anal. Ed., 1936, 8, 380). Residues determined by reaction with concentrated sulfuric acid, and colorimetric estimation of derivatives or by atomic absorption spectroscopy (AOAC Methods, 14th Ed., 3.020-3.028, 3.033-3.034, 3.013-3.016).
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 1500 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Moderate to minimal irritation to skin. Inhalation LC50 for rats 5.0 mg/l air. NOEL No chronic toxic effects have been observed in rats receiving 500 mg/kg copper in their diet. No record of occupational diseases attributable to copper. Other Sheep and calves are somewhat copper-sensitive, and livestock should not be allowed to graze on newly sprayed fields. Toxicity class WHO (a.i.) II EC classification Xn; R22
ECOTOXICOLOGY
Birds Not harmful; no significant history of ill-effect on birds. Fish LC50 (48 h) for young goldfish 60, adult goldfish 150, young guppies 50 mg/l. Daphnia LC50 (48 h) 18.9 mg/l. Bees LD50 >25 mg/bee. Worms Under conditions of moderate use and cultivation, the hazard to earthworms, and hence to soil structure, is insignificant.
ENVIRONMENTAL FATE
Animals Copper is an essential element and is under homeostatic control in mammals. Plants Plants resist copper accumulation and translocation to stems, leaves or seeds. Most plants growing on soils containing up to 1000 ppm copper showed only slight elevation in copper content compared to plants grown in normal soils. Soil/Environment Copper is strongly adsorbed to surfaces of minerals and organic matter, hence soil mobility is very low. In water, copper ions have a strong tendency to form complexes or to be adsorbed, followed by sedimentation. In the sediment, copper reacts with organic matter or sulfides; these reactions reduce bioavailability.
|