|
cumyluron
Herbicide
HRAC Z
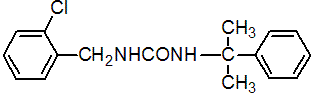
NOMENCLATURE
Common name cumyluron (BSI, pa E-ISO)
IUPAC name 1-(2-chlorobenzyl)-3-(1-methyl-1-phenylethyl)urea
Chemical Abstracts name N-[(2-chlorophenyl)methyl]-N'-(1-methyl-1-phenylethyl)urea
CAS RN [99485-76-4] Development codes JC-940 (Japan Carlit)
PHYSICAL CHEMISTRY
Composition >99.5% Mol. wt. 302.8 M.f. C17H19ClN2O Form White, odourless, crystalline solid. M.p. 166-167 °C B.p. 282?.5 °C/100.94 kPa V.p. 8.0 ´ 10-12 mPa (25 °C) KOW logP = 2.61 S.g./density 1.22 (20 °C) Solubility In water 0.879 mg/l (pH 6.7, 20.0?.5 °C). In acetone 11.0, methanol 14.4, hexane 0.00357, benzene 1.4, xylene 0.352 (g/l, 20.0?.5 °C). Stability Stable up to 150 °C. In water, DT50 1500 d (pH 5.0), 2830 d (pH 9.0).
COMMERCIALISATION
History Discovered by The Japan Carlit Co., Ltd; rights sold to Marubeni Corp. in 1996.
APPLICATIONS
Biochemistry Inhibitor of cell division or cell growth. Mode of action Absorbed by plant roots. Uses Pre-emergence herbicide for control of sedges (Cyperus spp.) in rice, at 700-1500 g/ha, and of annual bluegrass (Poa annua) in turf, at 1-2 ml/m2 (45% formulation). Formulation types GR; SC. Selected products: 'Gamyla' (rice) (Marubeni, Yashima); 'Mac 1' (turf) (Marubeni); mixtures: 'Kusabue' (+ pentoxazone) (Marubeni, Yashima, Nissan, Kaken); 'Habikoran' (+ thenylchlor+ benzofenap) (Yashima)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 2074, female rats 961 mg/kg. Skin and eye Acute dermal LD50 for rats and mice >2000 mg/kg. Inhalation LC50 for rats >6.21 mg/l. Other Non-carcinogenic, non-teratogenic.
ECOTOXICOLOGY
Birds Acute oral LC50 for quail >5620 ppm diet. Fish Acute LC50 (96 h) for carp >50, rainbow trout >10 ppm. Daphnia LC50 (24 h) for D. pulex >50 ppm. Algae EbC50 (72 h) for Selenastrum capricornutum >55mg/l. Bees LC50 (oral, in water) >200 ppm. Other beneficial spp. LC50 for silkworm >10 g/l.
|