|
clothianidin
Insecticide
IRAC 4A; neonicotinoid
NOMENCLATURE
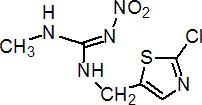
Common name clothianidin (BSI, pa ISO)
IUPAC name (E)-1-(2-chloro-1,3-thiazol-5-ylmethyl)-3-methyl-2-nitroguanidine
Chemical Abstracts name (E)-N-[(2-chloro-5-thiazolyl)methyl]-N'-methyl-N''-nitroguanidine
CAS RN [210880-92-5]; (formerly [205510-53-8]) Development codes TI-435 (Takeda)
PHYSICAL CHEMISTRY
Mol. wt. 249.7 M.f. C6H8ClN5O2S Form Colourless, odourless powder. M.p. 176.8 °C V.p. 1.3 ´ 10-4 mPa (25 °C, EEC A4). KOW logP = 0.7 (25 °C) Henry 2.9 ´ 10-11 Pa m3 mol-1 (20 °C) S.g./density 1.61 (20 °C) Solubility In water 0.304 (pH 4), 0.340 (pH 10) (both g/l, 20 °C). In heptane <0.00104, xylene 0.0128, dichloromethane 1.32, methanol 6.26, octanol 0.938, acetone 15.2, ethyl acetate 2.03 (all in g/l, 25 °C). pKa 11.09 (20 °C)
COMMERCIALISATION
History Discovered by Takeda Chemical Industries (now Sumitomo Chemical Takeda Agro Company Ltd) and under development jointly with Bayer AG. Reported by Y. Ohkawara et al., Proc. BCPC Conf. - Pests Dis., 2002, 1, 51 and M. Schwarz et al., ibid., 59. Manufacturers Bayer CropScience; Sumitomo Chemical Takeda
APPLICATIONS
Biochemistry Agonist of the nicotinic acetylcholine receptor, affecting the synapses in the insect central nervous system. Mode of action Translaminar and root systemic activity. Uses Soil, foliar, paddy and seed insecticide, under development for control of sucking and chewing insects in rice, fruit and vegetables, maize and rape. Formulation types FS. Selected products: 'Dantotsu' (Sumitomo Chemical Takeda); 'Poncho' (Bayer CropScience, Sumitomo Chemical Takeda); 'Clutch' (Arvesta)
OTHER PRODUCTS
'Fullswing' (Sumitomo Chemical Takeda) mixtures: 'Dantotsupadan' (+ cartap hydrochloride) (Sumitomo Chemical Takeda); 'Dantotsupadanvalida' (+ validamycin+ cartap hydrochloride) (Sumitomo Chemical Takeda); 'Delausdantotsu' (+ diclocymet) (Sumitomo Chemical Takeda); 'Hustler' (+ ferimzone+ phthalide+ validamycin+ cartap hydrochloride) (Sumitomo Chemical Takeda); 'Windantotsu' (+ carpropamid) (Sumitomo Chemical Takeda)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male and female rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for male and female rats >2000 mg/kg. Slightly irritating to eyes, not a skin irritant (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for male and female rats >6.1 mg/l. NOEL (2 y) for male rats 27.4, female rats 9.7 mg/kg b.w. daily; (1 y) for male dogs 7.8, female dogs 8.5 mg/kg b.w. daily. Other Not mutagenic. Not oncogenic in rats and mice. Not teratogenic in rats and rabbits.
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Dietary LC50 for bobwhite quail and mallard ducks >5200 ppm. Fish LC50 (96 h) for rainbow trout >100, carp >100, bluegill sunfish >120 mg/l. Daphnia EC50 (48 h) >120 mg/l. Algae ErC50 (72 h) for Scenedesmus subspicatus >270 mg/l. Bees Harmful to honeybees by direct contact, but no problems expected when not sprayed into flowering crops, or when used as a seed treatment. Worms LC50 (14 d) 13.2 mg/kg soil.
|