|
cloransulam-methyl
Herbicide
HRAC B WSSA 2; triazolopyrimidine
NOMENCLATURE
Common name cloransulam-methyl (BSI, pa ISO, ANSI)
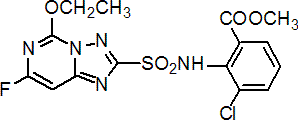
IUPAC name methyl 3-chloro-2-(5-ethoxy-7-fluoro[1,2,4]triazolo[1,5-c]pyrimidin-2-ylsulfonamido)benzoate; methyl 3-chloro-N-(5-ethoxy-7-fluoro[1,2,4]triazolo[1,5-c]pyrimidin-2-ylsulfonyl)anthranilate
Chemical Abstracts name methyl 3-chloro-2-[[(5-ethoxy-7-fluoro[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)sulfonyl]amino]benzoate
CAS RN [147150-35-4]; (acid [159518-97-5]) Development codes XDE-565 (Dow)
PHYSICAL CHEMISTRY
Mol. wt. 429.8; (acid 415.8) M.f. C15H13ClFN5O5S; (acid C14H11ClFN5O5S) Form Off-white powder. M.p. 216-218 °C V.p. 4.0 ´ 10-11 mPa (25 °C) KOW logP = 1.12 (pH 5), -0.365 (pH 7), -1.24 (pH 8.5), 0.268 (distilled water) S.g./density 1.538 (20 °C) Solubility In water 3 ppm (pH 5), 184 ppm (pH 7) (both 25 °C). In acetone 4360, acetonitrile 5500, dichloromethane 6980, ethyl acetate 980, methanol 470, hexane <10, octanol <10, toluene 14 (all in mg/l). Stability Stable to aqueous hydrolysis (pH 5), slowly degraded (pH 7), rapidly hydrolysed (pH 9). DT50 for aqueous photolysis 22 min. pKa 4.81 (20 °C)
COMMERCIALISATION
History First registered in USA in 1997. Manufacturers Dow AgroSciences
APPLICATIONS
Biochemistry Inhibits the acetolactate synthase (ALS) enzyme. The primary site of activity is in plant meristems. Soya beans quickly metabolise cloransulam-methyl into a non-active form; DT50 <5 h. Uses Applied to the soil surface or incorporated pre-emergence or post-emergence in soya beans, to control broad-leaved weeds. Applied by ground only, at 42-53 g/ha. Formulation types WG. Selected products: 'First Rate' (Dow AgroSciences); 'Meta' (Dow AgroSciences); 'Pacto' (Dow AgroSciences)
OTHER PRODUCTS
'Amplify' (Monsanto); 'Gangster' (Valent) mixtures: 'Frontrow' (+ flumetsulam) (Dow AgroSciences); 'Gauntlet' (+ sulfentrazone) (FMC)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Not a skin irritant (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >3.77 mg/l. NOEL (1 y) for dogs 5 mg/kg daily; (90 d) for male mice 50 mg/kg daily. ADI 0.1 mg/kg. Other Negative in mouse micronucleus and CHO tests. Toxicity class WHO (a.i.) U; EPA (formulation) III
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2250 mg/kg b.w. Dietary LC50 for bobwhite quail and mallard ducks >5620 ppm. Fish LC50 (96 h) for bluegill sunfish >295, rainbow trout >86 ppm. Daphnia LC50 (48 h) >163 mg/l. Algae EC50 for Selenastrum capricornutum 0.00346 ppm Other aquatic spp. LC50 (96 h) for grass shrimp (Palaemonetes pugio) >121 mg/l; LC50 (48 h) for Eastern oyster (Crassostrea virginica) >111 mg/l. Bees LD50 (48 h, contact) for honeybees >25 mg/bee. Worms NOEC (14 d) for earthworms 859 mg/kg soil. Other beneficial spp. Practically non-toxic.
ENVIRONMENTAL FATE
Animals In female rats, administered cloransulam-methyl is excreted mainly via the urine; in male rats, it is excreted in both urine and faeces. After 72 h, <0.1% of the dose was found in any tissue. Soil/Environment Rapidly photolysed in water, DT50 22 min (pH 7). Photolysis on soil surface, DT50 30-70 d (corrected for metabolism). The apparent transformation DT50 in aerobic soils 9-13 d (est.). The transformation DT50 of cloransulam-methyl residues, under anaerobic aquatic conditions, c. 16 d. Although cloransulam-methyl and its transformation products are likely to be only of short persistence on the surface, the chemicals may be mobile in soil and may leach into ground water. Residues are contained in the top 30 to 45 cm.
|