|
cinidon-ethyl
Herbicide
HRAC E WSSA 14; N-phenylphthalimide
NOMENCLATURE
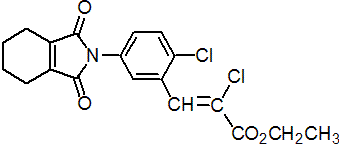
Common name cinidon-ethyl (BSI, pa ISO)
IUPAC name ethyl (Z)-2-chloro-3-[2-chloro-5-(1,2-cyclohex-1-enedicarboximido)phenyl]acrylate
Chemical Abstracts name ethyl (2Z)-chloro-3-[2-chloro-5-(1,3,4,5,6,7-hexahydro-1,3-dioxo-2H-isoindol-2-yl)phenyl]-2-propenoate
CAS RN [142891-20-1] ((Z)- isomer); [132057-06-8] (unspecified stereochemistry) Development codes BAS 615 H (BASF)
PHYSICAL CHEMISTRY
Composition Tech. is >90%. Mol. wt. 394.3 M.f. C19H17Cl2NO4 Form White, crystalline, odourless powder. M.p. 112.2-112.7 °C B.p. >360 °C V.p. <1 ´ 10-2 mPa (20 °C) KOW logP = 4.51 (25 °C) Henry <6.92 ´ 10-2 Pa m3 mol-1 (calc.) S.g./density 1.398 (20 °C) Solubility In water 0.057 mg/l (20 °C). In acetone 213, methanol 8, toluene 384 (all in g/l, 20 °C). Stability Rapidly hydrolysed and photolysed. Hydrolysis DT50 5 d (pH 5), 35 h (pH 7), 54 min (pH 9) (20 °C). Photolysis DT50 2.3 d (pH 5).
COMMERCIALISATION
History Launched by BASF in UK in 1998. Reported by W. Nuyken et al., Proc. Br. Crop Prot. Conf. - Weeds, 1999, 1, 81; see also references therein. Manufacturers BASF
APPLICATIONS
Biochemistry Protoporphyrinogen IX oxidase inhibitor. The accumulated protoporphyrinogen then acts as photosensitiser, promoting the formation of the radical oxygen species in the cells. This toxic agent causes lipid peroxidation which results in membrane disruption. These effects quickly lead to cell death and tissue disiccation. Mode of action Acts primarily by contact action, with no acropetal or basipetal translocation. Uses For post-emergence control of annual broad-leaved weeds, especially Galium aparine, Lamium spp., and Veronica spp., in winter and spring small grain cereals, at 50 g/ha. Phytotoxicity The most sensitive crop to pre-emergence application is sugar beet. Formulation types EC. Compatibility Known to be incompatible with diclofop-methyl, fenoxaprop-P-ethyl and difenzoquat metilsulfate. BASF recommend that cinidon-ethyl should be mixed only with isoproturon, chlormequat chloride and 'Duplosan' formulations. Selected products: 'Lotus' (BASF)
OTHER PRODUCTS
'Bingo' (BASF); 'Orbit' (BASF); 'Solar' (BASF); 'Vega' (BASF) mixtures: 'Lotus D' (+ 2,4-D-isoctyl) (BASF)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >2200 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Not a skin or eye irritant (rabbits). A skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >5.3 mg/l. NOEL (12 mo) for dogs 1 mg/kg b.w. daily. ADI 0.01 mg/kg.
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Fish LC50 (96 h) for rainbow trout >24.8 mg/l. Daphnia LC50 52.1 mg/l. Algae ErC50 for Pseudokirchneriella subcapitata 0.02, for Anabaena flos-aquae 1.53 mg/l. Other aquatic spp. ErC50 for Lemna gibba 0.602 mg/l. Bees LD50 (oral and contact) >200 mg/bee. Worms LC50 for Eisenia foetida >1000 mg/kg soil.
ENVIRONMENTAL FATE
Readily biodegradable in soil and water. Animals Following limited, but rapid, absorption, and widespread distribution in organs and tissues, the a.i. is extensively metabolised and rapidly excreted. Plants The a.i. is extensively metabolised; no bioavailable components of the residues present in grain are likely to be found at toxicologically significant levels. Soil/Environment Soil DT50 0.6-2 d (lab., aerobic conditions, 20 °C); rapidly mineralised. Rapidly degraded in water; decomposition increases with increasing alkalinity. Not stable photolytically in water.
|