|
chloridazon
Herbicide
HRAC C1 WSSA 5; pyridazinone (PSII)
NOMENCLATURE
Common name chloridazon (BSI, E-ISO); chloridazone ((f) F-ISO); pyrazon, a name formerly used by BSI and in many countries, is retained (ANSI, WSSA, Canada, Denmark, Poland); PAC (JMAF)
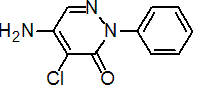
IUPAC name 5-amino-4-chloro-2-phenylpyridazin-3(2H)-one
Chemical Abstracts name 5-amino-4-chloro-2-phenyl-3(2H)-pyridazinone
Other names PCA CAS RN [1698-60-8] (formerly [58858-18-7]) EEC no. 216-920-2 Development codes BAS 119H (BASF)
PHYSICAL CHEMISTRY
Mol. wt. 221.6 M.f. C10H8ClN3O Form Colourless, odourless solid; (tech., brown, almost odourless solid). M.p. 206 ºC; (tech., 198-202 ºC) V.p. <0.01 mPa (20 ºC) KOW logP = 1.19 (pH 7) Henry <6.52 ´ 10-6 Pa m3 mol-1 (calc.) S.g./density 1.54 (20 ºC). Solubility In distilled water 0.34 g/l (20 ºC). In methanol 15.1, ethyl acetate 3.7, dichloromethane 1.9, toluene 0.1 (all in g/l, 20 ºC). Practically insoluble in n-heptane. Stability Stable up to 50 ºC for ³2 years. Stable in aqueous media at pH 3-9. DT50 in simulated sunlight (xenon lamp, 2100 mEinstein m-2 s-1) 150 h (pH 7, water).
COMMERCIALISATION
History Herbicide reported by A. Fischer (Weed Res., 1962, 2, 177). Introduced by BASF AG in 1964. Patents US 3222159; US 3210353; DE 1105232 Manufacturers BASF; Istrochem; Oxon
APPLICATIONS
Biochemistry Photosynthetic electron transport inhibitor at the photosystem II receptor site. Mode of action Selective systemic herbicide, rapidly absorbed by the roots, with translocation acropetally to all plant parts. Uses Control of annual broad-leaved weeds in sugar beet, fodder beet and beetroot, by application pre-plant incorporated, pre-emergence, or post-emergence at 1.3-3.25 kg/ha. Often used in combination with other herbicides. Phytotoxicity Phytotoxic to e.g. cabbage, carrots, cucumbers, lima beans and tomatoes. Formulation types SC; WG; WP. Selected products: 'Flirt' (BASF); 'Pyramin' (BASF); 'Betozon' (Sipcam); 'Burex' (Istrochem, Agricola); 'Erbitox Bietole' (Siapa); 'Trojan' (Bayer CropScience); mixtures: 'Fiesta' (+ quinmerac) (sugar beet) (BASF); 'Rebell' (+ quinmerac) (BASF)
OTHER PRODUCTS
'Expander' (BASF); 'Takron' (BASF); 'Better' (Sipcam); 'Clorimag' (Tecomag); 'Gladiator DF' (Tripart); 'Sculptor' (Sipcam UK) mixtures: 'Magnum' (+ ethofumesate) (BASF); 'Pyracur' (+ metolachlor) (BASF); 'Pyradex T' (+ tri-allate) (BASF); 'Advizor S' (+ lenacil) (DuPont); 'Ashlade CP' (+ propachlor) (Nufarm UK); 'Gremlin' (+ ethofumesate) (Sipcam UK); 'Lenapac' (+ lenacil) (Hokko, Maruwa); 'Spectron' (+ ethofumesate) (Bayer CropScience) Discontinued products: 'Questar' * (BASF); 'Gladiator' * (Tripart); 'Herbon Silver' * (Atlas Interlates); 'Silver' * (Atlas Interlates); 'Starter' * (Truchem); 'Weedmaster' * (Portman) mixtures: 'Pyradex' * (+ di-allate) (BASF)
ANALYSIS
Product analysis by i.r. spectrometry or by rplc (CIPAC Handbook,1988, D, 31; ibid., 1998, H, 76). Residues in plants determined by glc or hplc (Rückstandanalytik von Pflanzenschutzmitteln,1987, Verlag Chemie, 9, Lieferung 89-A-1).
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 3830, female rats 2140, male mice 2860, female mice 3100 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin and eyes (rabbits). Inhalation LC50 (4 h) for rats >5.4 mg/l air. NOEL (2 y) for rats 16, mice 152 mg/kg b.w. daily. ADI 0.16 mg/kg. Other Non-mutagenic, non-teratogenic, non-carcinogenic. Toxicity class WHO (a.i.) U; EPA (formulation) III EC classification R43| N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg b.w. Dietary LC50 for bobwhite quail >5000, mallard ducks 4260 mg/kg diet. Fish LC50 (96 h) for trout 32-46, bluegill sunfish 93 mg/l. Daphnia LC50 (48 h) 132 mg/l. Algae EC50 (120 h) for Chlorella fusca 1.9 mg/l. Bees Not toxic to bees; LD50 (48 h, oral and contact) >200 mg/bee. Worms LC50 (14 d) for Eisenia foetida 1050 ppm.
ENVIRONMENTAL FATE
Animals In rats, following a single oral application, 85% is excreted in the urine within 1 hour, and 13% in the faeces. After repeated oral applications, excretion proceeds similarly, with 75% in the urine and 15% in the faeces. Plants In beet, conjugation to form the N-glucosyl metabolite occurs. Soil/Environment In soil, microbial degradation involves splitting off the phenyl group, to give 5-amino-4-chloropyridazin-3(2H)-one, which is not herbicidally-active (A. Fischer, Weed Res., 1962, 2, 177-184). Persists in soil for c. 6-8 weeks under sufficiently moist conditions. Koc 89-340.
|