|
chinomethionat
Fungicide, acaricide
NOMENCLATURE
Common name chinomethionat (E-ISO); chinométhionate ((m) F-ISO); quinomethionate (BSI); oxythioquinox (Australia, ESA); quinoxalines (JMAF); no name (USA)
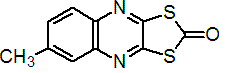
IUPAC name S,S-(6-methylquinoxaline-2,3-diyl) dithiocarbonate; 6-methyl-1,3-dithiolo[4,5-b]quinoxalin-2-one
Chemical Abstracts name 6-methyl-1,3-dithiolo[4,5-b]quinoxalin-2-one
CAS RN [2439-01-2] EEC no. 219-455-3 Development codes Bayer 36 205; Bayer SAS 2074 Official codes ENT 25 606
PHYSICAL CHEMISTRY
Mol. wt. 234.3 M.f. C10H6N2OS2 Form Yellow crystals. M.p. 170 ºC V.p. 0.026 mPa (20 ºC) KOW logP = 3.78 (20 ºC) Henry 6.09 ´ 10-3 Pa m3 mol-1 (calc.) S.g./density 1.556 (20 °C) Solubility In water 1 mg/l (20 ºC). In toluene 25, dichloromethane 40, hexane 1.8, isopropanol 0.9, cyclohexanone 18, dimethylformamide 10, petroleum oils 4 (all in g/l, 20 ºC). Soluble in hot benzene and dioxane. Stability Relatively stable under normal conditions. Hydrolysed in alkaline media; DT50 (22 ºC) 10 d (pH 4), 80 h (pH 7), 225 min (pH 9).
COMMERCIALISATION
History Acaricide and fungicide reported by K. Sasse (Hoefchen-Briefe (Engl. Ed.), 1960, 13, 197; K. Sasse et al., Angew. Chem., 1960, 72, 973). Introduced by Bayer AG. Patents DE 1100372; BE 580478 Manufacturers Bayer CropScience
APPLICATIONS
Mode of action Selective non-systemic contact fungicide with protective and eradicant action. Uses Control of powdery mildews and spider mites on fruit (including citrus), ornamentals, cucurbits, cotton, coffee, tea, tobacco, walnuts, vegetables, and glasshouse crops; American gooseberry mildew on gooseberries and currants. Phytotoxicity Phytotoxic to certain varieties of apple, pear, currant, rose, and ornamentals. Formulation types DP; FU; SC; WP. Compatibility Incompatible with mineral oils (phytotoxicity may result), and with formulations based on thiram. Selected products: 'Morestan' (Bayer CropScience)
ANALYSIS
Product analysis by hplc (CIPAC Handbook, 1985, 1C, 2019; AOAC Methods, 17th Ed., 986.08) or by u.v. spectrometry (details available from Bayer CropScience). Residues determined by glc (Pestic. Anal. Man., 1979, I, 201-I, II; Man. Pestic. Residue Anal., 1987, I, S13; A. Ambrus et al., J. Assoc. Off. Anal. Chem., 1981, 64, 733; R. T. Krause & E. M. August, ibid., 1983, 66, 1018), or by colorimetry after conversion to a derivative (H. Tietz et al., ibid., 1962, 15, 166; C. A. Anderson, Anal. Methods Pestic., Plant Growth Regul. Food Addit., 1967, 5, 277). Methods for the determination of residues are also available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 50, 52 (see part 2 of the Bibliography). Oral Acute oral LD50 for male rats 2541, female rats 1095 mg/kg. Skin and eye Acute percutaneous LD50 for rats >5000 mg/kg. Slightly irritating to skin; severely irritating to eyes (rabbits). Inhalation LC50 (4 h) for male rats >4.7, female rats 2.2 mg/l (dust). NOEL (2 y) for rats 40, male mice 270, female mice <90 mg/kg diet; (1 y) for dogs 25 mg/kg diet. ADI (JMPR) 0.006 mg/kg b.w. [1987]. Toxicity class WHO (a.i.) III; EPA (formulation) III EC classification R62| Xn; R20/21/22, R48/22| Xi; R36| R43| N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail 196 mg/kg. Dietary LC50 (5 d) for bobwhite quail 2409, mallard ducks >5000 mg/kg diet. Fish LC50 (96 h) for bluegill sunfish 0.0334, rainbow trout 0.131, golden orfe 0.24 mg/l. Daphnia LC50 (48 h) 0.12 mg/l. Algae ErC50 (96 h) for Scenedesmus 0.14 mg/l. Bees Not toxic to bees; LD50 >100 mg/bee. Worms LC50 (14 d) >1000 mg/kg.
ENVIRONMENTAL FATE
Animals In rats, following oral administration, chinomethionat is rapidly metabolised, and c. 90% is eliminated within 3 days in the faeces and urine. The main metabolite is chinomethionat acid (dimethylmercaptoquinoxaline-6-carboxylic acid), which also occurs in the conjugated form. Plants After application to fruit, no penetration of the a.i. or metabolites in the fruit pulp was observed. The only metabolite detected was dihydromethylquinoxalinedithiol. Soil/Environment Koc 45-90 (3 soil types from sandy loam to high organic matter). DT50 in standard soil land 2 1-3 d.
|