|
carboxin
Fungicide
FRAC 7, C2; carboxamide
NOMENCLATURE
Common name carboxin (BSI, E-ISO, ANSI); carboxine ((f) F-ISO); carbathiin (Canada); no name (Denmark, Germany)
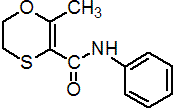
IUPAC name 5,6-dihydro-2-methyl-1,4-oxathi-ine-3-carboxanilide
Chemical Abstracts name 5,6-dihydro-2-methyl-N-phenyl-1,4-oxathiin-3-carboxamide
CAS RN [5234-68-4] EEC no. 226-031-1 Development codes D 735
PHYSICAL CHEMISTRY
Composition Tech. grade is >97% pure. Mol. wt. 235.3 M.f. C12H13NO2S Form White crystals; (tech. is a pale yellow powder with a slight sulfurous odour) M.p. 91-92 °C (pure a.i.) V.p. 0.020 mPa (25 ºC) KOW logP = 2.3 Henry 3.24 ´ 10-5 Pa m3 mol-1 (calc.) S.g./density 1.45 Solubility In water 0.147 g/l (20 °C). In acetone 221.2, methanol 89.33, ethyl acetate 107.7 (g/l, 20 °C). Stability Stable to hydrolysis at pH 5, pH 7 and pH 9 (25 ºC). In aqueous solutions exposed to light, DT50 1.54 h (pH 7, 25 °C). pKa <0.5
COMMERCIALISATION
History Fungicide reported by B. von Schmeling & M. Kulka (Science, 1966, 152, 659). Introduced by Uniroyal Chemical Co., Inc. (now Crompton Corp.) in 1969. Patents US 3249499; US 3393202; US 3454391 Manufacturers Crompton; Fengle; Hindustan; Jin Hung; Kemira FC; Sundat
APPLICATIONS
Biochemistry Inhibits mitochondrial function by disrupting complex II (succinate dehydrogenase) in the respiratory electron transport chain. Mode of action Systemic fungicide. Uses Seed treatment for control of smuts and bunts (particularly loose smut, Ustilago spp.), at 50-200 g/100 kg seed, on barley, wheat, and oats; seedling diseases (particularly Rhizoctonia spp.) of barley, wheat, oats, rice, cotton, peanuts, soya beans, vegetables, maize, sorghum, and other crops. The dimorphic forms do not differ in fungicidal activity. Formulation types SC; FS; WP; Seed treatment. Compatibility Not compatible with pesticides which are highly alkaline or acidic. Selected products: 'Vitavax' (Crompton); 'Hiltavax' (Hindustan); 'Kemikar' (Kemira FC); mixtures: 'Anchor' (+ thiram) (Crompton); 'Vitavax 200FF' (+ thiram) (Crompton)
OTHER PRODUCTS
'Oxatin' (Diachem) mixtures: 'Vitaflo Extra' (+ imazalil+ thiabendazole) (Crompton); 'Vitavax Extra' (+ imazalil+ thiabendazole) (Crompton); 'Enhance' (+ gamma-HCH+ maneb) (Gustafson); 'Kick Start' (+ diazinon+ gamma-HCH) (Helena); 'Stiletto' (+ metalaxyl+ thiram) (Trace); 'Vitavax CT' (+ thiram) (Helena); 'Vitavax M DC' (+ captan) (Helena); 'Vitavax M' (+ thiram) (Helena); 'Zaprawa Oxafun T' (+ thiram) (Azot) Discontinued products mixtures: 'Vitavax RS' * (+ gamma-HCH+ thiram) (Uniroyal); 'Vitavax-Plus' * (+ thiabendazole) (Uniroyal); 'Vitavax-R' * (+ thiram) (Uniroyal)
ANALYSIS
Product analysis by hplc or gc; details available from Crompton. Residue analysis by gc-msd; details available from Crompton. Drinking water analysis by hplc; details available from Crompton.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 2864 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >4000 mg/kg. Non-irritating to eyes and skin (rabbits). Inhalation LC50 (4 h) for rats >4.7 mg/l air. NOEL NOEL for chronic toxicity (2 y) for rats 1 mg/kg b.w. daily. ADI 0.01 mg/kg. Toxicity class WHO (a.i.) U; EPA (formulation) III ('Vitavax 200FF')
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail 3302 mg/kg. Dietary LC50 (8 d) for mallard ducks and bobwhite quail >5000 ppm. Fish LC50 (96 h) for rainbow trout 2.3, bluegill sunfish 3.6 mg/l. Daphnia EC50 (48 h) >57 mg/l. Algae EC50 (5 d) for Pseudokirchneriella subcapitata 0.48 mg/l. Other aquatic spp. EC50 (14 d) for Lemna 0.92 mg/l. Bees Not hazardous to bees when used as directed; LD50 >181 mg/bee. Worms LC50 (14 d) for earthworms 500-1000 ppm.
ENVIRONMENTAL FATE
Animals Extensively metabolised in rats and eliminated mainly in urine and to a lesser extent faeces. Major metabolites in urine are p-hydroxylated carboxin sulfoxide, 4-acetamidophenol and its O-glucuronide. Plants Undergoes oxidation to carboxin sulfoxide and carboxin sulfone. Soil/Environment Soil DT50 <1 d (20 °C). Koc 71.
|