|
carbetamide
Herbicide
HRAC K2 WSSA 23; carbamate (mi)
NOMENCLATURE
Common name carbetamide (BSI, E-ISO, (m) F-ISO, ANSI, WSSA); no name (Germany)
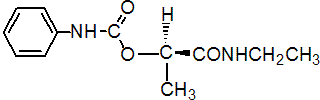
IUPAC name (R)-1-(ethylcarbamoyl)ethyl carbanilate
Chemical Abstracts name (R)-N-ethyl-2-[[(phenylamino)carbonyl]oxy]propanamide
CAS RN [16118-49-3] EEC no. 240-286-6 Development codes 11 561 RP (Rhône-Poulenc)
PHYSICAL CHEMISTRY
Mol. wt. 236.3 M.f. C12H16N2O3 Form Colourless crystals. M.p. 119 ºC; (tech., >110 ºC) V.p. Negligible (20 ºC) Solubility In water c. 3.5 g/l (20 ºC). In acetone 900, dimethylformamide 1500, ethanol 850, methanol 1400, cyclohexane 0.3 (all in g/l). Stability Stable under normal storage conditions.
COMMERCIALISATION
History Herbicide reported by J. Desmoras et al. (C. R. Journ. Etud. Herbic. Conf. COLUMA, 2nd, 1963, p. 14). Introduced by Rhône-Poulenc Agrochimie (now Bayer CropScience), who sold it to Feinchemie Schwebda GmbH in 2000. Patents GB 959204; BE 597035; US 3177061 Manufacturers Feinchemie Schwebda
APPLICATIONS
Biochemistry Mitosis inhibitor (microtubule organisation). Mode of action Selective herbicide, absorbed principally by the roots, and also by the leaves. Uses Control of annual grasses (including volunteer cereals) and some broad-leaved weeds, at 2 kg/ha, in clover, alfalfa, sainfoin, brassicas, field beans, peas, lentils, sugar beet, oilseed rape, chicory, endive, sunflowers, caraway, strawberries, vines, and fruit orchards. Formulation types EC; WP. Selected products: 'Legurame' (Feinchemie Schwebda); mixtures: 'Pradone' (+ dimefuron) (Feinchemie Schwebda)
OTHER PRODUCTS
'Carbetamex' (Feinchemie Schwebda) mixtures: 'Helmsman' (+ diflufenican+ oxadiazon) (Bayer CropScience) Discontinued products: 'Ronstar TX' * (Feinchemie Schwebda)
ANALYSIS
Product analysis by hplc (CIPAC Handbook, 1992, E, 28) or by titration of the ethylamine liberated on hydrolysis (J. Desmoras et al., Anal. Methods Pestic. Plant Growth Regul., 1973, 7, 509). Residues determined by hydrolysis to aniline, which is measured by colorimetry of a derivative (idem, ibid.). Details of chromatographic methods are available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >2000, mice 1720, dogs 900 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >500 mg/kg. Non-irritating to eyes (rabbits). Inhalation LC50 (4 h) for rats >0.13 mg/l air. NOEL In 90 d feeding trials, no effect observed with rats receiving 3200 mg/kg diet, or dogs receiving 12 800 mg/kg diet. Toxicity class WHO (a.i.) U; EPA (formulation) IV
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Fish LC50 (96 h) for rainbow trout and common carp >100 mg/l. Daphnia EC50 (48 h) 36.5 mg/l. Bees Not hazardous to bees when used as directed. Worms LC50 600 mg/kg soil.
ENVIRONMENTAL FATE
Plants Rapidly metabolised, leaving no residues in the plant. Soil/Environment Microbially degraded in soil; DT50 c. 1 mo. Duration of activity at low temperatures is c. 2-3 mo. Kd ranges from 0.10 (0.01% o.m., pH 6.6) to 7.92 (16.9% o.m., pH 6.8) (H. J. Pedersen et al., Pestic. Sci., 44, 131 (1995)).
资料收集库(DocBook) 小笨象工作室 /www.9ele.com/
|