|
cafenstrole
Herbicide
HRAC K3 WSSA 15
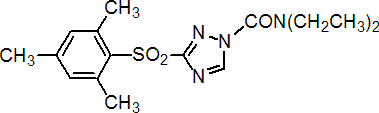
NOMENCLATURE
Common name cafenstrole (pa ISO)
IUPAC name N,N-diethyl-3-mesitylsulfonyl-1H-1,2,4-triazole-1-carboxamide
Chemical Abstracts name N,N-diethyl-3-[(2,4,6-trimethylphenyl)sulfonyl]-1H-1,2,4-triazole-1-carboxamide
CAS RN [125306-83-4] Development codes CH-900 (Chugai)
PHYSICAL CHEMISTRY
Mol. wt. 350.4 M.f. C16H22N4O3S Form Colourless crystals. M.p. 114-116 °C V.p. 5.3 ´ 10-5 mPa (20 ºC) KOW logP = 3.21 S.g./density 1.30 (25 °C) Solubility In water 2.5 mg/l (20 ºC). Stability Stable in neutral and weakly acidic media. Relatively stable to heat.
COMMERCIALISATION
History Discovered in 1987; reported by Chugai Pharmaceutical Co., Ltd (agrochemical interests now Eiko Kasei Co., Ltd) (M. Kanzaki et al., Proc. Br. Crop Prot. Conf. - Weeds, 1991, III, 923 (and refs. therein)). Introduced in Japan in 1997 by Eiko Kasei Co., Ltd. and transferred to SDS Biotech KK in 2001. Patents JP 1858798; US 5147445; EP 0332133 Manufacturers SDS Biotech KK
APPLICATIONS
Biochemistry Inhibits cell division. Uses Pre- and early post-emergence control of Echinochloa oryzicola, Cyperus difformis and other annual weeds in paddy rice, at 150-300 g/ha. Formulation types GR; SC; WG; WP. Selected products: 'Grachitor' (SDS Biotech KK)
OTHER PRODUCTS
Mixtures: 'Crush' (+ daimuron+ imazosulfuron) (Sumitomo Chemical Takeda); 'Die-Hard' (+ pyrazosulfuron-ethyl) (Nissan, Yashima); 'Joystar' (+ cyhalofop-butyl+ daimuron+ bensulfuron-methyl) (Kumiai); 'Joyster' (+ cyhalofop-butyl+ daimuron+ bensulfuron-methyl) (Kumiai); 'Kusatory Ace Jumbo' (+ daimuron+ bensulfuron-methyl) (Sankyo Agro); 'Nebiros' (+ cyclosulfamuron+ daimuron) (BASF); 'Niceshot-Jumbo' (+ pyrazolynate+ bromobutide) (Sankyo Agro); 'Rakudar' (+ daimuron+ bensulfuron-methyl) (Sankyo Agro); 'Redstar' (+ cyhalofop-butyl+ daimuron+ halosulfuron-methyl) (Nissan); 'Sanattack' (+ ethoxysulfuron) (Sankyo Agro); 'Striker' (+ cyhalofop-butyl+ pyrazosulfuron-ethyl) (Nissan, Yashima); 'Technostar' (+ pyrazosulfuron-ethyl) (Nissan); 'Weedless A36' (+ daimuron+ azimsulfuron+ bensulfuron-methyl) (Sankyo Agro, DuPont, Eiko Kasei, SDS Biotech KK); 'Weedless' (+ daimuron+ bensulfuron-methyl) (Sankyo Agro)
ANALYSIS
By hplc with u.v. detection.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats and mice >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Inhalation LC50 (14 d) for rats >1.97 g/m3. ADI 0.003 mg/kg. Other Non-mutagenic in the Ames test.
ECOTOXICOLOGY
Birds Acute oral LD50 for quail and mallard ducks >2000 mg/kg. Fish LC50 (48 h) for carp >1.2 mg/l. Daphnia LC50 (3 h) >500 mg/kg. Bees Acute oral LC50 (72 h) for bees >1000 ppm; contact LC50 (72 h) >5000 ppm.
ENVIRONMENTAL FATE
Animals The major metabolite in the rat and dog is 3-(2,4,6-trimethylphenylsulfonyl)-1,2,4-triazole. Plants The major metabolite in rice is the same as in the rat. Soil/Environment DT50 (Japanese paddy) c. 7 d, (Japanese upland) c. 8 d.
|