|
cadusafos
Nematicide, insecticide
IRAC 1B; organophosphate
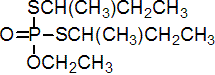
NOMENCLATURE
Common name cadusafos (BSI, draft E-ISO, ANSI)
IUPAC name S,S-di-sec-butyl O-ethyl phosphorodithioate
Chemical Abstracts name O-ethyl S,S-bis(1-methylpropyl) phosphorodithioate
Other names ebufos* (rejected common name proposal) CAS RN [95465-99-9] Development codes FMC 67 825
PHYSICAL CHEMISTRY
Mol. wt. 270.4 M.f. C10H23O2PS2 Form Colourless to yellow liquid. B.p. 112-114 ºC/0.8 mmHg V.p. 1.2 ´ 102 mPa (25 ºC) KOW logP = 3.9 S.g./density 1.054 (20 ºC) Solubility In water 248 mg/l. Completely miscible with acetone, acetonitrile, dichloromethane, ethyl acetate, toluene, methanol, isopropanol, and heptane. Stability Stable up to 50 ºC. Half-life in light <115 d. F.p. 129.4 ºC (Seta closed cup)
COMMERCIALISATION
History Nematicide and insecticide discovered by FMC Corp. Manufacturers FMC
APPLICATIONS
Biochemistry Cholinesterase inhibitor. Mode of action Contact and stomach action. Uses Controls various nematodes and larvae of Noctuidae, Agriotes spp. and other soil insects in bananas, citrus, maize, potatoes, sugar cane, tobacco and vegetables, at 3-10 kg/ha. Formulation types EW; GR. Selected products: 'Apache' (FMC); 'Rugby' (FMC)
OTHER PRODUCTS
Discontinued products: 'Taredan' * (FMC)
ANALYSIS
Product analysis by glc. Residues determined by glc with FID. Details available from FMC Corp.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 62, 64 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats 37.1, mice 71.4 mg tech./kg. Skin and eye Acute percutaneous LD50 for male rabbits 24.4, female rabbits 41.8 mg/kg. Non-irritating to skin and practically non-irritating to eyes of rabbits. Inhalation LC50 (4 h) for rats 0.026 mg/l air. NOEL (2 y) for rats 1 mg/kg diet; (1 y) for male dogs 0.001, female dogs 0.005 mg/kg daily. In oncogenicity tests (2 y), male mice 0.5, female mice 1 mg/kg diet. ADI (JMPR) 0.0003 mg/kg [1991]. Toxicity class WHO (a.i.) Ib; EPA (formulation) III
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail 16, mallard ducks 230 mg/kg. Fish LC50 (96 h) for rainbow trout 0.13, bluegill sunfish 0.17 mg/l. Daphnia LC50 (48 h) 1.6 mg/l. Algae EC50 (96 h) 5.3 mg/l. Worms LC50 (14 d) for Eisenia foetida 72 mg/kg.
ENVIRONMENTAL FATE
EHC 63 (WHO, 1986; a general review of organophosphorus insecticides). Animals Readily absorbed, metabolised and eliminated in urine and faeces. Hydroxy sulfones were the major metabolites, followed by phosphorothioic and sulfonic acids. Soil/Environment DT50 in silty clay, sandy loam soils 11-55 d. Koc 144-351.
|