|
butroxydim
Herbicide
HRAC A WSSA 1; cyclohexanedione oxime
NOMENCLATURE
Common name butroxydim (BSI, pa E-ISO)
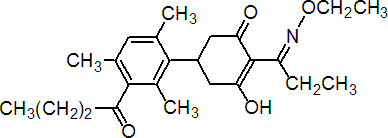
IUPAC name 5-(3-butyryl-2,4,6-trimethylphenyl)-2-(1-ethoxyiminopropyl)-3-hydroxycyclohex-2-enone
Chemical Abstracts name 2-[1-(ethoxyimino)propyl]-3-hydroxy-5-[2,4,6-trimethyl-3-(1-oxobutyl)phenyl]-2-cyclohexen-1-one
CAS RN [138164-12-2] Development codes ICIA0500 (ICI)
PHYSICAL CHEMISTRY
Mol. wt. 399.5 M.f. C24H33NO4 Form Off-white powdery solid. M.p. 80.8 °C V.p. 1 ´ 10-3 mPa (20 °C) KOW logP = 1.90 (pH 7, 25 °C) Henry 5.79 ´ 10-5 Pa m3 mol-1 (calc.) S.g./density 1.20 (tech., 25 °C) Solubility In water 6.9 mg/l (20 °C). In dichloromethane >500, acetone 450, toluene 480, acetonitrile 380, methanol 90, hexane 30 (all in g/l). Stability Hydrolysis DT50 10.5 d (pH 5), >240 d (pH 7), stable (pH 9) (all 25 °C). pKa 4.36 (23 °C), weak acid
COMMERCIALISATION
History First sold in 1995. Divested to Crop Care Australasia Pty Ltd in 2002.
APPLICATIONS
Biochemistry Fatty acid synthesis inhibitor, by inhibition of acetyl CoA carboxylase (ACCase). Mode of action Post-emergence herbicide. Uses A post-emergence grass herbicide for use on broad-leaved crops, at 25-75 g/ha. Formulation types WG. Selected products: 'Falcon' (Crop Care)
ANALYSIS
Residues determined by hplc with u.v. detection, or by hplc-ms.
MAMMALIAN TOXICOLOGY
Oral LD50 for male rats 3476, female rats 1635 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Moderate skin irritant; mild eye irritant (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >2.99 mg/l. NOEL NOAEL (1 y) for dogs 5 mg/kg b.w. daily; NOEL (2 y) for rats 2.5, mice 10 mg/kg b.w. daily (liver tumours only in males at high dose); NOEL developmental for rats 5, rabbits 15 mg/kg b.w. daily. ADI 0.025 mg/kg. Other Genotoxicity negative. Not oncogenic (rats). Toxicity class WHO (a.i.) III EC classification (T; R61| R22| R38| N; R50, R53)
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >2000, bobwhite quail 1221 mg/kg. Sub-acute dietary LC50 (5 d) for mallard ducks >5200, bobwhite quail 5200 mg/kg. Fish LC50 (96 h) for rainbow trout >6.9, bluegill sunfish 8.8 mg/l. Daphnia LC50 (48 h) >3.7 mg/l. Algae EbC50 for Selenastrum capricornutum 0.71 mg/l. Bees LD50 (contact, 24 h) >200 mg/bee. Worms LC50 (14 d) for Eisenia foetida >1000 mg/kg.
ENVIRONMENTAL FATE
Animals In rats, following oral administration, >90% of the dose is excreted within 7 d. Various oxidative transformations of the butyryl chain dominate the metabolic pathway. Neither parent nor metabolites bioaccumulate in tissues. Plants Rapidly metabolised in plants. Soil/Environment Soil Koc 6-1270 (stronger adsorption in low pH soils). Rapid degradation in aerobic soil, lab. DT50 c. 9 d (20 °C, 40% MHC, pH 7.0, 4.09% o.m.); metabolites include 5-(3-butyryl-2,4,6-trimethylphenyl)-3-hydroxy-2-(1-iminopropyl)cyclohex-2-enone, 6-(3-butyryl-2,4,6-trimethylphenyl)-2-ethyl-4,5,6,7-tetrahydro-4-oxo-1,3-benzoxazole, 2-(3-butyryl-2,4,6-trimethylphenyl)glutaric acid and 5-(3-butyryl-2,4,6-trimethylphenyl)-3-hydroxy-2-propionylcyclohex-2-enone.
|