|
bromopropylate
Acaricide
benzilate
NOMENCLATURE
Common name bromopropylate (BSI, E-ISO, (m) F-ISO, ANSI, ESA); phenisobromolate (JMAF)
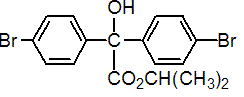
IUPAC name isopropyl 4,4'-dibromobenzilate
Chemical Abstracts name 1-methylethyl 4-bromo-a-(4-bromophenyl)-a-hydroxybenzeneacetate
CAS RN [18181-80-1] EEC no. 242-070-7 Development codes GS 19 851 (Geigy) Official codes ENT 27 552
PHYSICAL CHEMISTRY
Mol. wt. 428.1 M.f. C17H16Br2O3 Form White crystals. M.p. 77 ºC V.p. 6.8 ´ 10-3 mPa (20 ºC) KOW logP = 5.4 Henry <5.82 ´ 10-3 Pa m3 mol-1 (calc.) S.g./density 1.59 at 20 ºC Solubility In water <0.5 mg/l (20 ºC). In acetone 850, dichloromethane 970, dioxane 870, benzene 750, methanol 280, xylene 530, isopropanol 90 (all in g/kg, 20 ºC). Stability Fairly stable in neutral or slightly acidic media; DT50 34 d (pH 9).
COMMERCIALISATION
History Acaricide reported by H. Grob et al. (Abstr. Int. Congr. Plant Prot. 6th, 1967, p. 198). Introduced by J. R. Geigy S.A. (now Syngenta AG). Patents GB 1178850; BE 691105; CH 471065 Manufacturers Syngenta
APPLICATIONS
Mode of action Non-systemic acaricide with contact action, and long residual activity. Uses Control of all stages of tetranychid and eriophyid mites on pome fruit, stone fruit, citrus fruit, vines, strawberries, hops, cotton, soya beans, cucurbits, vegetables, and ornamentals; applied at 25-50 g/hl on citrus, pome fruits, stone fruits, grape, tea, vegetables, ornamentals and at 500-750 g/ha on cotton. Also used to control parasitic mites in beehives. Phytotoxicity Slightly phytotoxic to certain varieties of apple, plum, and ornamentals. Formulation types EC. Selected products: 'Neoron' (Syngenta)
OTHER PRODUCTS
'Acarol' (Syngenta); 'Folbex VA' (Syngenta)
ANALYSIS
Residues determined by glc with ECD (M. A. Luke et al., J. Assoc. Off. Anal. Chem., 1981, 64, 1187). Methods reviewed by J. L. Daft in Comp. Analyt. Profiles, Chapter 11.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 68, 70 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats >5000 mg tech./kg. Skin and eye Acute percutaneous LD50 for rats >4000 mg/kg. Slightly irritating to skin; non-irritating to eyes (rabbits). Inhalation LC50 for rats >4000 mg/kg. NOEL (2 y) for rats 500 mg/kg diet (c. 25 mg/kg daily); (1 y) for mice 1000 mg/kg diet (c. 143 mg/kg daily). ADI (JMPR) 0.03 mg/kg b.w. [1993]. Toxicity class WHO (a.i.) III (company classification); EPA (formulation) IV
ECOTOXICOLOGY
Birds Acute oral LD50 for Japanese quail >2000 mg/kg. Dietary LC50 (8 d) for Pekin ducks 600, Japanese quail 1000 mg/kg diet. Fish LC50 (96 h) for rainbow trout 0.35, bluegill sunfish 0.5, carp 2.4 mg/l. Daphnia LC50 (48 h) 0.17 mg/l. Algae EC50 (72h) for Scenedesmus subspicatus >52 mg/l. Bees Not toxic to bees; LC50 (24 h) 183 mg/bee. Worms LC50 (14 d) for earthworms >1000 mg/kg soil. Other beneficial spp. Safe on the relevant adult and immature stages of anthocorids, mirids, coccinellids, Chrysoperla, Hemerobius, staphylinids, carabids, syrphids and dolichopodids in deciduous fruits, citrus and hops. The potential hazard to predatory mites can be minimised by avoiding early season sprays.
ENVIRONMENTAL FATE
Animals Bromopropylate is rapidly and efficiently eliminated in animals. Metabolism occurs by cleavage of the isopropyl ester and, to a minor extent, by oxidation. Metabolites formed after oxidation were 3-hydroxybenzilate and conjugates. Plants Studies with 14C-labelled bromopropylate showed little penetration into leaves or fruit. Degradation was slow. Soil/Environment The principal metabolite in soil is 4,4-dibromobenzilic acid. For details of persistence in soil, see J. Environ. Qual., 1973, 2, 115. DT50 c. 40-70 d (lab. and field studies). Low mobility in soil.
|