|
bispyribac-sodium
Herbicide
HRAC B WSSA 2; pyrimidinyloxybenzoic
NOMENCLATURE
Common name bispyribac-sodium (BSI, pa ISO)
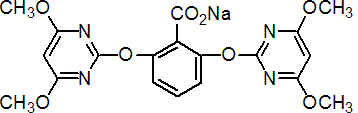
IUPAC name sodium 2,6-bis(4,6-dimethoxypyrimidin-2-yloxy)benzoate
Chemical Abstracts name sodium 2,6-bis[(4,6-dimethoxy-2-pyrimidinyl)oxy]benzoate
CAS RN [125401-75-4] for acid; [125401-92-5] for sodium salt Development codes KIH-2023; KUH-911 (both Kumiai); V-10029
PHYSICAL CHEMISTRY
Composition Tech. is >93%. Mol. wt. 452.4 M.f. C19H17N4NaO8; C19H18N4O8 for the acid Form White powder. M.p. 223-224 °C V.p. 5.05 ´ 10-6 mPa (25 ºC) KOW logP = -1.03 (23 °C) Henry 3.12 ´ 10-11 Pa m3 mol-1 (calc.) S.g./density Bulk density 0.0737 (20 °C, CIPAC MT 3) Solubility In water 73.3 g/l (25 ºC). In methanol 26.3, acetone 0.043 g/l (25 °C). Stability Stable in water; DT50 >1 y (pH 7 & 9), 448 h (pH 4). Stable to light. Not decomposed after 14 d at 55 °C. pKa 3.05
COMMERCIALISATION
History Discovered in 1988. Reported by Kumiai Chemical Industry Co., Ltd (M. Yokoyama et al., Proc. Br. Crop Prot. Conf. - Weeds, 1993, 1, 61). Developed jointly by Kumiai and Ihara Chemical Industry Co., Ltd and introduced in 1997. Patents US 4906285; EP 321846 Manufacturers Ihara/Kumiai
APPLICATIONS
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor. Mode of action Selective, systemic post-emergence herbicide, absorbed by foliage and roots. Uses Control of grasses, sedges and broad-leaved weeds, especially Echinochloa spp., in direct-seeded rice, at rates of 15-45 g/ha. Also used to stunt growth of weeds in non-crop situations. Formulation types AL; SC (water-soluble liquid). Selected products: 'Grass-short' (non-crop land) (Kumiai); 'Nominee' (Kumiai); 'Short-keep' (non-crop land) (Riken Green)
OTHER PRODUCTS
'Regiment' (Valent)
ANALYSIS
By hplc. Details available from Kumiai.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 4111, female rats 2635, male and female mice 3524 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin; slightly irritating to eyes (rabbits). Inhalation LC50 (4 h) for rats >4.48 mg/l. NOEL (2 y) for male rats 20 mg/kg diet (1.1 mg/kg b.w. daily), female rats 20 mg/kg diet (1.4 mg/kg b.w. daily), male mice 14.1 mg/kg daily, female mice 1.7 mg/kg b.w. daily. ADI 0.011 mg/kg. Other Non-mutagenic in the Ames test, and non-teratogenic to rats and rabbits. Toxicity class WHO (a.i.) U
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2250 mg/kg. Dietary LC50 (5 d) for bobwhite quail and mallard ducks >5620 mg/kg diet. Fish Acute LC50 (96 h) for rainbow trout and bluegill sunfish >100 ppm. Daphnia LC50 (48 h) >100 ppm. Algae EC50 (120 h) for Selenastrum capricornutum 3.4 mg/l, NOEC 0.625 mg/l. Bees LD50 (oral) >200 mg/bee; LC50 (contact) >7000 mg/l spray. Worms NOEL (14 d) for Eisenia foetida >1000 mg/kg soil.
ENVIRONMENTAL FATE
Animals >95% of dose applied to rats was excreted in urine and faeces within 7 d. Plants Following application to foliage and soil at 5-leaf stage of rice plants, c. 10% of carbon label was distributed between straw and roots at harvest. Soil/Environment In soil, DT50 <10 d (flooded and upland conditions).
|