|
bilanafos
See also The BioPesticide Manual, 2nd Ed., entry 2:102
Herbicide
HRAC H WSSA 10; phosphinic acid
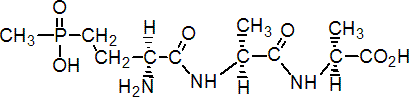
NOMENCLATURE
bilanafos
Common name bilanafos (BSI, draft E-ISO, (m) draft F-ISO); bialaphos (JMAF)
IUPAC name 4-[hydroxy(methyl)phosphinoyl]-L-homoalanyl-L-alanyl-L-alanine
Other names bialaphos CAS RN [35597-43-4] Development codes MW-801; SF-1293
bilanafos-sodium
Common name bilanafos-sodium
IUPAC name sodium 4-[hydroxy(methyl)phosphinoyl]-L-homoalanyl-L-alanyl-L-alaninate
Chemical Abstracts name (2S)-2-amino-4-(hydroxymethylphosphinyl)butanoyl-L-alanyl-L-alanine, monosodium salt
CAS RN [71048-99-2] Development codes SF-1293Na; MW-851
PHYSICAL CHEMISTRY
bilanafos
Mol. wt. 323.3 M.f. C11H22N3O6P Solubility In water 1 kg/l. In methanol 500, ethanol 250 (both in g/l). Practically insoluble in organic solvents such as acetone, benzene, butan-1-ol, chloroform, diethyl ether, hexane. Stability Unstable in strong acids and strong alkalis. Specific rotation [a]D25 -34?(10 g/l water)
bilanafos-sodium
Mol. wt. 345.3 M.f. C11H21N3NaO6P Form Colourless crystals. M.p. c. 160 ºC (decomp.) Solubility In water >1000 g/l. In acetic acid 3.2, methanol 0.31 g/l; practically insoluble in organic solvents such as ethanol, acetone, chloroform and benzene. Stability Stable to light and heat. Unstable in strong acids and strong alkalis.
COMMERCIALISATION
Production Bilanafos is produced by fermentation of Streptomyces hygroscopicus. History Herbicidal activity of this fermentation product reported by K. Tachibana et al. (Abstr. 5th Int. Congr. Pestic. Chem., IVa, Abstract 19) and the compound reviewed by S. Mase (Jpn. Pestic. Inf., 1984, 45, 27). Introduced by Meiji Seika Kaisha Ltd. in 1984. Manufacturers Meiji Seika
APPLICATIONS
Biochemistry Inhibits glutamine synthetase activity, and causes accumulation of ammonia and inhibition of photosynthesis. Mode of action Non selective contact herbicide, absorbed by the foliage, with some systemic activity. Inactivated on contact with soil.
bilanafos-sodium
Uses Post-emergence control of wide range of annual and perennial broad-leaved weeds and grasses, in vegetable fields, orchards, vineyards, pre-planting in paddy fields, and non-crop land. Formulation types SL. Selected products: 'Herbie' (Meiji Seika, Hokko, Sumitomo Chemical Takeda)
OTHER PRODUCTS
bilanafos-sodium
Discontinued products: 'Herbiace' * (Meiji Seika)
ANALYSIS
Product by nmr. Residues by glc. Details from Meiji Seika.
MAMMALIAN TOXICOLOGY
bilanafos
Toxicity class WHO (a.i.) II
bilanafos-sodium
Reviews A. Suzuki et al., J. Pesticide Sci., 1987, 12, 347. Oral Acute oral LD50 for male rats 268, female rats 404 mg/kg. Skin and eye Acute percutaneous LD50 for rats >3000 mg/kg. Non-irritating to skin and eyes (rabbits). Inhalation LC50 for male rats 2.57, female rats 2.97 mg/l. Other In sub-acute and chronic toxicity studies, no ill-effects were observed. Non-carcinogenic, non-mutagenic and non-teratogenic. Not mutagenic in Ames and Rec assays.
ECOTOXICOLOGY
bilanafos-sodium
Birds Acute oral LD50 for chickens >5000 mg/kg. Fish LC50 (48 h) for carp >1000 mg/l. Daphnia LC50 (3 h) >5000 mg/l. Worms Not toxic to earthworms. Other beneficial spp. Not toxic to soil microorganisms.
ENVIRONMENTAL FATE
Animals In mice, the main metabolite in the faeces following oral administration was glufosinate (q.v.) (A. Suzuki et al., J. Pestic. Sci., 1987, 12, 105). Soil/Environment Rapidly degraded in soil and water.
|