|
bifenox
Herbicide
HRAC E WSSA 14; diphenyl ether
NOMENCLATURE
Common name bifenox (BSI, draft E-ISO, (m) draft F-ISO, ANSI, WSSA)
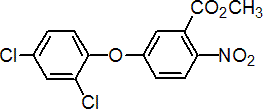
IUPAC name methyl 5-(2,4-dichlorophenoxy)-2-nitrobenzoate
Chemical Abstracts name methyl 5-(2,4-dichlorophenoxy)-2-nitrobenzoate
CAS RN [42576-02-3];formerly [12680-11-4] EEC no. 255-894-7 Development codes MC-4379 (Mobil); MCTR-1-79; MCTR-12-79
PHYSICAL CHEMISTRY
Composition ³97% pure. Mol. wt. 342.1 M.f. C14H9Cl2NO5 Form Yellow crystals with a slightly aromatic odour. M.p. 84-86 ºC V.p. 0.32 mPa (30 ºC) KOW logP = 4.5 Henry 1.14 ´ 10-2 Pa m3 mol-1 S.g./density 0.65 g/ml (bulk density) Solubility In water 0.35 mg/l (25 ºC). In acetone 400, chlorobenzene 400, xylene 300, ethanol <50 (all in g/kg, 25 ºC). Slightly soluble in aliphatic hydrocarbons. Stability Thermally stable up to 175 ºC; total decomposition occurs above 290 ºC. Stable in slightly acidic or slightly alkaline media, but rapidly hydrolysed above pH 9. DT50 in saturated aqueous solution, 24 min at 250-400 nm; c. 5 h for a thin film on soil.
COMMERCIALISATION
History Herbicide reported by W. M. Dest et al. (Proc. Northeast. Weed Sci. Conf., 1973, 27, 31). Introduced by Mobil Chemical Co. Agrochemical Division (now Bayer CropScience) and later by Eli Lilly & Co. (agrochemical interests now Dow AgroSciences, who no longer manufacture or market it). Patents GB 1232368; US 3652645; US 3776715 all to Mobil Manufacturers Feinchemie Schwebda
APPLICATIONS
Biochemistry Protoporphyrinogen oxidase inhibitor. Acts by cellular membrane disruption and by inhibition of photosynthesis. Mode of action Selective herbicide, absorbed by the foliage, emerging shoots and roots, with limited translocation from roots and foliage to shoots. Uses Control of annual broad-leaved weeds and some grasses in cereals, maize, sorghum, soya beans, rice, and some other crops at 0.75-1 kg/ha. Applied pre-plant incorporated, pre-emergence, or directed post-emergence. Often used in combination with other herbicides to extend the spectrum of activity. Formulation types SC; EC; GR; WP. Selected products: 'Foxpro' (Feinchemie Schwebda); 'Modown' (Feinchemie Schwebda); 'Tolkan Fox' (Feinchemie Schwebda)
OTHER PRODUCTS
'Athlet' (Feinchemie Schwebda); 'Bifenix' (Feinchemie Schwebda); 'Exel' (Feinchemie Schwebda); 'Foxtar' (Feinchemie Schwebda); 'Foxtril' (Feinchemie Schwebda) mixtures: 'Fizz' (+ ioxynil+ pyraflufen-ethyl) (ioxynil as a salt) (Bayer CropScience); 'Foxpro D+' (+ ioxynil+ mecoprop-P) (Feinchemie Schwebda); 'Foxtril P' (+ ioxynil+ mecoprop-P) (ioxynil and mecoprop-P as potassium salts) (Bayer CropScience); 'Kalao' (+ ioxynil+ mecoprop) (Sipcam Phyteurop); 'Milan' (+ pyraflufen-ethyl) (Nihon Nohyaku, Feinchemie Schwebda) Discontinued products mixtures: 'Sirocco' * (+ MCPA+ mecoprop-P) (Bayer); 'Quickstep' * (+ dicamba) (Novartis)
ANALYSIS
Product analysis is by rplc (CIPAC Handbook, 1995, G, 11-17). Residues determined by glc with MCD. Details available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Reviews Toxicological Evaluation of the Herbicide Bifenox, J-O. Säfwenberg, Säfwenberg Consulting, Surbrunnsgatan 43 A, S-11348, Stockholm, Sweden. Oral Acute oral LD50 for rats >5000 mg tech./kg, mice 4556 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Non-irritating to skin and eyes. Inhalation LC50 for rats >0.91 mg/l air. NOEL (2 y) for rats 80, dogs 145, mice 30 mg/kg b.w. daily. Other Non-mutagenic in mouse lymphoma assay and Ames test. Non-teratogenic. Toxicity class WHO (a.i.) U; EPA (formulation) IV
ECOTOXICOLOGY
Birds Dietary LC50 (8 d) for ducks and pheasants >5000 mg/kg diet. Fish LC50 (96 h) for rainbow trout >0.67, bluegill sunfish >0.27 mg/l. Daphnia LC50 (48 h) 0.66 mg/l. Other aquatic spp. LC50 (96 h) for Palaemonetes pugio (grass shrimp) 569 ppm, for Mysidopsis bahia (mysid shrimp) 0.065 mg/l; (48 h) for Crassostrea virginica (Eastern oyster) 210 mg/l. Bees LD50 (contact) >1000 mg/bee. Worms EC50 and NOEC >1000 mg/kg.
ENVIRONMENTAL FATE
Animals Bifenox is relatively rapidly absorbed and eliminated from the body; 5-(2,4-dichlorophenyl)-2-nitrobenzoic acid was the major urinary metabolite, with no bifenox detected. Bifenox together with 5-(2,4-dichlorophenyl)anthranilate were detected in faeces. Soil/Environment DT50 in soil c. 5-7 d. Duration of residual activity is c. 7-8 w. Degradation is mainly chemical and microbial, and the two metabolites are 5-(2,4-dichlorophenoxy)-2-nitrobenzoic acid and methyl 5-(2,4-dichlorophenoxy)anthranilate. Mobile in soil; Kd 50-300; Koc 500-23 000.
|