|
bifenazate
Acaricide
NOMENCLATURE
Common name bifenazate (ANSI, BSI proposed)
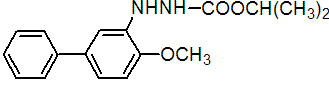
IUPAC name isopropyl 3-(4-methoxybiphenyl-3-yl)carbazate
Chemical Abstracts name 1-methylethyl 2-(4-methoxy[1,1'-biphenyl]-3-yl)hydrazinecarboxylate
CAS RN [149877-41-8] Development codes D2341; NC-1111 (Nissan)
PHYSICAL CHEMISTRY
Composition Tech. is >95% m/m. Mol. wt. 300.4 M.f. C17H20N2O3 Form White, odourless crystals; (tech. is a beige solid). M.p. 123-125 °C (pure a.i.) V.p. <1 ´ 10-2 mPa (25 °C) KOW logP = 3.4 (25 °C, pH 7) Henry 1 ´ 10-3 Pa m3 mol-1 S.g./density 1.31 Solubility In water 2.06 mg/l (20 °C). In acetonitrile 95.6, ethyl acetate 102, methanol 44.7, toluene 24.7, hexane 0.232 g/l. Stability Stable for >1 y at 20 °C and 50% r.h. DT50 (hydrolysis) 9.10 (pH 4), 5.4 (pH 5), 0.8 (pH 7), 0.08 (pH 9) (all in d, 25 °C); DT50 (photolysis) 17 h (25 °C, pH 5). pKa 12.94 (23 °C) F.p. ³110 °C (Setaflash) Other properties Surface tension 64.9 mN/m (22 ºC).
COMMERCIALISATION
History Reported by M. A. Dekeyser et al. (Proc. Br. Crop Prot. Conf. - Pests Dis., 1996, 2, 487). Discovered by Uniroyal Chemical Co., Inc. (now part of Crompton Corp.). Developed jointly by Uniroyal and Nissan Chemical and first marketed in 2000. Patents US 5367093; US 5438123 Manufacturers Crompton
APPLICATIONS
Mode of action Non-systemic acaricide with predominantly contact action and long residual action. Uses Under development by Crompton Corp. for control of phytophagous mites (both eggs and motile stages) on crops including citrus, tree fruits, vines, hops, nuts, vegetables, ornamentals, cotton and maize. Proposed use rates are 0.15-0.75 kg/ha. Formulation types SC; WG; WP. Selected products: 'Acramite' (Crompton); 'Floramite' (Crompton)
OTHER PRODUCTS
'Mito-kohne' (Nissan)
ANALYSIS
Product analysis by rp hplc with OCECD.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Very slight skin irritation, slight eye irritation (rabbits); not classified as a skin or eye irritant according to EPA or EU criteria. Not a guinea pig skin sensitiser (Buehler); is a skin sensitiser (Magnusson & Kligman). Inhalation LC50 for rats >4.4 mg/l. NOEL NOEL (90 d) for rats 40 ppm (2.7 and 3.2 mg/kg daily for males and females respy.), dogs 40 ppm (0.9 and 1.3 mg/kg daily for males and females respy.). NOEL (1 y) for dogs 40 ppm (1.014 and 1.051 mg/kg daily for males and females, respy.). NOEL (2 y) for rats 20 ppm (1.0 and 1.2 mg/kg daily for males and females, respy.). NOEL (78 week) for mice 10 ppm (1.5 and 1.9 mg/kg daily for males and females, respy.). ADI 0.01 mg/kg. Other Negative in Ames test, not mutagenic, not teratogenic in rats and rabbits. Not carcinogenic to rats and mice.
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail 1142 mg/kg. Dietary LC50 (5 d) for bobwhite quail 2298, mallard ducks 726 mg/kg diet. Fish LC50 (96 h) for bluegill sunfish 0.58, rainbow trout 0.76 mg/l. Daphnia EC50 (48 h) 0.50 mg/l. Algae ErC50 (96 h) for Selenastrum capricornutum 0.90 mg/l. Bees LD50 (48 h, oral) >100 mg/bee; (contact) 8.5 mg/bee. Worms LC50 (14 d) >1250 mg/kg. Other beneficial spp. Harmless to predacious mites such as the phytoseiids Amblyseius fallacis, Galendromus occidentalis and Zetzellia mali. 'Harmless' to Chrysoperla carnea, Orius variegatus. Encarsia formosa and Poecilus cupreus (IOBC).
ENVIRONMENTAL FATE
Animals In animals, the product is considered to be of poor bioavailability, and most of the dose is excreted in the faeces. Absorption is dose dependent (80-85% at 10 mg/kg, 22-29% at 1000 mg/kg). The absorbed dose undergoes oxidation to the corresponding azo compound, and hydroxylation; hydroxylated metabolites appear in the urine as sulfate or glucuronide conjugates. Plants Considered to be non-systemic; most residues stay on the surface and peel of the crops, where it is mostly not metabolised. Traces that penetrated the peel were metabolised as for animals. Soil/Environment DT50 in aerobic soil c. 7 h; DT50 (anaerobic) c. <1 d. Neither bifenazate nor its degradation products leached in a variety of soil types; Koc (by hplc) 1778. DT50 in natural water 45 min.; DT50 for field dissipation £5 d.
|