|
beta-cypermethrin
Insecticide
IRAC 3; pyrethroid
NOMENCLATURE
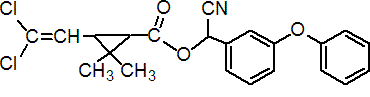
Common name beta-cypermethrin (BSI, draft E-ISO)
IUPAC name Roth: A reaction mixture comprising two enantiomeric pairs in ratio c. 2:3 (S)-a-cyano-3-phenoxybenzyl (1R)-cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate and
(R)-a-cyano-3-phenoxybenzyl (1S)-cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
with (S)-a-cyano-3-phenoxybenzyl (1R)-trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate and
(R)-a-cyano-3-phenoxybenzyl (1S)-trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
Chemical Abstracts name cyano(3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate; 2 parts of enantiomer pair [(1R)-1a(S*),3a] and [(1S)-1a(R*),3a] with 3 parts of enantiomer pair [(1R)-1a(S*),3b] and [(1S)-1a(R*),3b]
Other names asymethrin*(rejected common name proposal) CAS RN [52315-07-8] (undefined stereochemistry, also used for cypermethrin); [72204-43-4] ([(1S)-1a(R*),3a] isomer; Roth: (S) (1R)-cis- isomer); [65731-84-2] ([(1R)-1a(S*),3a] isomer; Roth: (R) (1S)-cis- isomer); [83860-31-5] ([(1S)-1a(R*),3b] isomer; Roth: (S) (1R)-trans- isomer); [65732-07-2] ([(1R)-1a(S*),3b] isomer; Roth: (R) (1S)-trans- isomer) EEC no. 276-457-7 (S) (1R)-cis- isomer; 265-898-0 (R) (1S)-cis- isomer; 281-086-9 (S) (1R)-trans- isomer; 265-896-6 (R) (1S)-trans- isomer Development codes CHINOIN 0619200; AT0IABO3 Official codes OMS 3068
PHYSICAL CHEMISTRY
Composition A mixture consisting of two enantiomeric pairs in ratio 2:3: (S) (1R)-cis and (R) (1S)-cis with (S) (1R)-trans and (R) (1S)-trans. Tech. grade beta-cypermethrin contains ³95% (normally >97%) relevant stereoisomers. Mol. wt. 416.3 M.f. C22H19Cl2NO3 Form Tech. forms colourless or pale yellow crystals. M.p. 63.1-69.2 ºC (varies with even a small - 1% - change in isomer ratio) B.p. 286.1?.06 ºC/97.4 kPa V.p. 1.8 ´ 10-4 mPa (20 ºC) KOW logP = 4.7 ?.04 S.g./density 1.336?.0050 (20 ºC) Solubility In water 51.5 (5 ºC), 93.4 (25 ºC), 276.0 (35 ºC) (all in mg/l, pH 7). In isopropanol 11.5, xylene 349.8, dichloromethane 3878, acetone 2102, ethyl acetate 1427, petroleum ether 13.1 (all in mg/ml, 20 ºC). Stability Stable to 150 ºC; to air and sunlight; in neutral and slightly acidic media; epimerised in presence of base, hydrolysed in strongly alkaline media. DT50 (extrapolated) 50 d (pH 3, 5, 6), 40 d (pH 7), 20 d (pH 8), 15 d (pH 9) (all at 25 ºC).
COMMERCIALISATION
History Insecticide introduced in Hungary (1989) by Chinoin Pharmaceutical & Chemical Works Co., Ltd (now Agro-Chemie Pesticide Manufacturing Trading and Distributing Ltd). Patents HU 198612; EP 208758; US 4963584 Manufacturers Agro-Chemie; Fengle; Jiangsu Yangnong
APPLICATIONS
Biochemistry Acts on the nervous system of insects, disturbs the function of neurons by interaction with the sodium channel. Mode of action Non-systemic insecticide with contact and stomach action. Uses It can be used against a wide range of insect pests in public health (e.g. flies, cockroaches, mosquitoes, fleas, lice, bugs) and in veterinary applications (ectoparasitic ticks and mites). In plant protection, it is effective against Coleoptera and Lepidoptera and gives good protection against Orthoptera, Diptera, Hemiptera, and Homoptera. Mainly used in alfalfa, cereals, cotton, grapes, maize, oilseed rape, pome fruit, potatoes, soya beans, sugar beet, tobacco and vegetables. Formulation types CS; EC; ME; SC; UL; gel. Selected products: 'Chinmix' (Agro-Chemie); 'Peststop-B' (Bábolna); mixtures: 'Chintop' (+ quinalphos) (Agro-Chemie)
OTHER PRODUCTS
'Chinmix Turbo' (with synergist) (Agro-Chemie)
ANALYSIS
Product analysis by hplc or glc; analysis of pyrethroids reviewed by E. Papadopoulou-Mourkidou in Comp. Analyt. Profiles. Residues determined by glc. Details available from Agro-Chemie.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for female rats 166, male rats 178, female mice 48, male mice 43 mg/kg. Skin and eye Acute percutaneous LD50 for rats >5000 mg/kg; mild skin and eye irritant (rabbits); non-sensitising to skin (guinea pigs). Inhalation LC50 (4 h) for rats >1.97 mg/l. NOEL (2 y) for rats 250 mg/kg diet; (90 d) for rats 100 mg/kg diet. Not teratogenic in rats or rabbits. In 3-generation reproduction study, NOEL for rats 350 mg/kg. In 2 y carcinogenicity study, NOEL 500 mg/kg diet. ADI 0.05 mg/kg. Other Not mutagenic in Ames, SCE and micronucleus assays. Toxicity class WHO (a.i.) II (company classification)
ECOTOXICOLOGY
Birds Acute oral LD50 for quail 8030, pheasants 3515 mg 5% a.i. formulation/kg. LC50 (8 d) for pheasants and quail >5000 mg 5% a.i. formulation/kg diet. Fish LC50 (96 h) for carp 0.028, catfish 0.015, grass carp 0.035 mg 5% a.i. formulation/l. Fish are not harmed under normal field conditions. Daphnia LC50 (96 h) 0.00026 mg 5% a.i. formulation/l. Algae LC50 for Selenastrum capricornutum 56.2 mg/l. Bees LD50 (oral, 48 h) 0.0018 mg a.i./bee (as 5% formulation); (contact, 24 h) 0.085 l/ha (as 5% formulation); but not harmful at normal rate under field conditions.
ENVIRONMENTAL FATE
Soil/Environment Soil DT50 10 d (lime-furred chernozom, pH 6.96). DT50 in water 1.2 d.
|