|
benthiavalicarb-isopropyl
Fungicide
FRAC U1, H2; amino acid amide carbamate
NOMENCLATURE
Common name benthiavalicarb-isopropyl (BSI proposed)
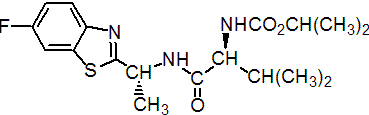
IUPAC name isopropyl [(S)-1-{[(R)-1-(6-fluoro-1,3-benzothiazol-2-yl)ethyl]carbamoyl}-2-methylpropyl]carbamate
Chemical Abstracts name 1-methylethyl [(1S)-1-[[[(1R)-1-(6-fluoro-2-benzothiazolyl)ethyl]amino]carbonyl]-2-methylpropyl]carbamate
CAS RN [177406-68-7]; [413615-35-7] (acid) Development codes KIF-230; KUF-1001
PHYSICAL CHEMISTRY
Mol. wt. 381.5 M.f. C18H24FN3O3S Form White powder. M.p. 152.0 °C V.p. <3.0 ´ 10-1 mPa (25 °C) KOW logP = 2.52 Henry 8.72 ´ 10-3 Pa m3 mol-1 S.g./density 1.25 (20.5 °C) Solubility In water 13.14 mg/l (20 °C). Stability Stable in water; DT50 >1 y (25 °C, pH 4, 7, and 9).
COMMERCIALISATION
History Developed jointly by Kumiai Chemical and Ihara Chemical Industry Co., Ltd. Patents US 5789428 Manufacturers Ihara/Kumiai
APPLICATIONS
Biochemistry Proposed inhibitor of cell wall synthesis. Mode of action Protective and curative fungicide. Uses For use against downy mildew and late blight on vegetables, potatoes and grapes, at 25-75 g/ha. Formulation types WG.
ANALYSIS
Details available from Kumiai.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Inhalation LC50 for rats >4.6 mg/l. NOEL NOEL (24 month) for male rats 9.9 mg/kg, for female rats 12.5 mg/kg.
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Fish LC50 for carp, bluegill, and rainbow trout >10 mg/l. Daphnia LC50 >10 mg/l. Algae ErC50 >100 mg/l. Bees LD50 for honeybees >100 mg/bee. Worms LC50 >1000 ppm.
ENVIRONMENTAL FATE
Animals In rats, following oral administration, excretion was predominantly via the bile and virtually completed by 168 hours. The metabolism was complex; the predominant routes were glutathione conjugation, or hydroxylation on the benzene or the valyl moieties. Plants In plants, metabolism proceeds slowly. The major metabolites are similar to those in animals. Soil/Environment In the lab., benthiavalicarb-isopropyl was readily degraded in soil; DT50 11-19 d (20 °C, aerobic), 40 d (20 °C, anaerobic). Koc 121-258.
|