|
benomyl
Fungicide
FRAC 1, B1; benzimidazole
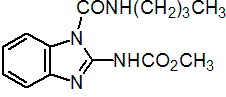
NOMENCLATURE
Common name benomyl (BSI, E-ISO, (m) F-ISO, ANSI, JMAF)
IUPAC name methyl 1-(butylcarbamoyl)benzimidazol-2-ylcarbamate
Chemical Abstracts name methyl [1-[(butylamino)carbonyl]-1H-benzimidazol-2-yl]carbamate
CAS RN [17804-35-2] EEC no. 241-775-7 Development codes T 1991 (DuPont)
PHYSICAL CHEMISTRY
Mol. wt. 290.3 M.f. C14H18N4O3 Form Colourless crystals. M.p. 140 ºC (decomp.) V.p. <5.0 ´ 10-3 mPa (25 ºC) KOW logP = 1.37 Henry <4.0 ´ 10-4 (pH 5); <5.0 ´ 10-4 (pH 7); <7.7 ´ 10-4 (pH 9) (all in Pa m3 mol-1, calc.) S.g./density Bulk density 0.38 Solubility In water 3.6 (pH 5), 2.9 (pH 7), 1.9 (pH 9) (all in mg/l, room temperature). In chloroform 94, dimethylformamide 53, acetone 18, xylene 10, ethanol 4, heptane 0.4 (all in g/kg, 25 ºC). Stability Hydrolysis DT50 3.5 h (pH 5), 1.5 h (pH 7), <1 h (pH 9) (all 25 °C). In some solvents, dissociates to form carbendazim and butyl isocyanate (M. Chiba & E. A. Cherniak, J. Agric. Food Chem., 1978, 26, 573). Solubility in water and stability at various pH values investigated by R. P. Singh & M. Chiba (ibid., 1985, 33, 63). Stable to light. Decomposed on storage in contact with water and under moist conditions in soil. Mechanism of the acid-catalysed decomposition in aqueous media, J. P. Calmon & D. R. Sayag(ibid., 1976, 24, 314, 317).
COMMERCIALISATION
History Fungicide reported by C. J. Delp & H. L. Klopping (Plant Dis. Rep., 1968, 52, 95). Introduced by E. I. du Pont de Nemours and Co. in 1970, who ceased marketing it in 2001. Patents NL 6706331; US 3631176 Manufacturers Agro-Chemie; Aragro; CAC; Gilmore; Jiangsu Eternal; Sannong; Sharda; Sinon; Sundat
APPLICATIONS
Biochemistry Inhibits mitosis by binding to beta-tubuline. Mode of action Systemic fungicide with protective and curative action. Absorbed through the leaves and roots, with translocation principally acropetally. Uses Effective against a wide range of Ascomycetes, and Fungi Imperfecti and some Basidiomycetes in cereals, grapes, pome and stone fruit, rice and vegetables. It is also effective against mites, primarily as an ovicide. Also used as pre-harvest sprays or dips for the control of storage rots of fruit and vegetables. Typical rates are: on field and vegetable crops, 140-550 g a.i./ha; on tree crops 550-1100 g/ha; for post-harvest uses 25-200 g/hl. Phytotoxicity Non-phytotoxic if used as directed. Russetting is possible with Golden Delicious apples. Formulation types WP. Compatibility Incompatible with alkaline materials. Selected products: 'Cekumilo' (Cequisa); 'Fundazol' (Agro-Chemie); 'Gilomyl' (Gilmore); 'Pilarben' (Pilarquim); 'Romyl' (Rotam); 'Viben' (Vipesco)
OTHER PRODUCTS
'Benex' (Crystal) mixtures: 'Benlate T' (+ thiram) (Hokko); 'Viben-C' (+ copper oxychloride) (Vipesco) Discontinued products: 'Benlate' * (DuPont); 'Agrocit' * (Agro-Chemie); 'Benor' * (Aragro); 'Benzole' * (Agro Chemicals)
ANALYSIS
Product analysis by i.r. spectrometry (F. J. Baude et al., Anal. Methods Pestic. Plant Growth Regul., 1978, 10, 157) or by rplc (AOAC Methods, 17th Ed., 984.09; CIPAC Handbook, 1988, D, 14). Residues determined by cation-exchange hplc (J. J. Kirkland et al., J. Agric. Food Chem., 1973, 21, 368; N. Aharonson & A. Ben-Aziz, J. Assoc. Off. Anal. Chem., 1973, 56, 481; J. E. Farrow et al., Analyst (London), 1977,102, 752). Benomyl can be converted to a derivative and differentiated from carbendazim by hplc (M. Chiba & R. P. Singh, J. Agric. Food Chem., 1986, 34, 108).
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 74, 76 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats >5000 mg a.i./kg. Skin and eye Acute percutaneous LD50 for rabbits >5000 mg/kg; negligible irritant to skin, temporary to eyes (rabbits). Inhalation LC50 (4 h) for rats >2 mg/l air. NOEL (2 y) for rats >2500 mg/kg diet (maximum rate tested), no evidence of histopathological changes; for dogs 500 mg/kg diet. ADI (JMPR) 0.1 mg/kg b.w. [1995]; residues should be compared against the ADI for carbendazim; environmental assessment also performed. Toxicity class WHO (a.i.) U; EPA (formulation) IV EC classification R68
ECOTOXICOLOGY
Birds Dietary LC50 (8 d) for mallard ducks and bobwhite quail >10 000 mg/kg diet (using 50% formulation). Fish LC50 (96 h) for rainbow trout 0.27, goldfish 4.2 mg/l. LC50 (48 h) for guppy 3.4 mg/l. Daphnia LC50 (48 h) 640 mg/l. Algae EbC50 (72 h) 2.0 mg/l, (120 h) 3.1 mg/l. Bees Not toxic to bees. LD50 (contact) >50 mg/bee. Worms LC50 (14 d) 10.5 mg/kg.
ENVIRONMENTAL FATE
EHC 148 (WHO, 1993). EHC 148 concludes that, although benomyl is highly toxic to aquatic organisms, this effect is unlikely to be seen in the field due to the low bioavailability of sediment-bound residues. Earthworm populations may take more than two years to recover following field application. Animals In animals, the butylcarbamoyl group is removed to give the relatively stable carbendazim, followed by slow degradation to non-toxic 2-aminobenzimidazole. Hydroxylation also occurs, and the principal metabolite 5-hydroxybenzimidazole carbamate is converted to the O- and N-conjugates; other possible metabolites include 4-hydroxy-2-benzimidazolemethylcarbamate. Benomyl and its metabolites are excreted in the urine and faeces within a few days, with no accumulation in animal tissue. Plants In plants, the butylcarbamoyl group is removed to give the relatively stable carbendazim, followed by slow degradation to non-toxic 2-aminobenzimidazole. Further degradation involves cleavage of the benzimidazole nucleus. Benomyl per se is stable on the surface of banana skins (J. Cox et al., Pestic. Sci., 1974, 5, 135; J. Cox & J. A. Pinegar, ibid., 1976, 7, 193). Soil/Environment Benomyl is rapidly converted to carbendazim in the environment, DT50 2 and 19 h in water and in soil, respectively. Data from studies on both benomyl and carbendazim are therefore relevant for the evaluation of environmental effects. Koc 1900.
|