|
beflubutamid
Herbicide
HRAC F1 WSSA 12
NOMENCLATURE
Common name beflubutamid (BSI, pa ISO)
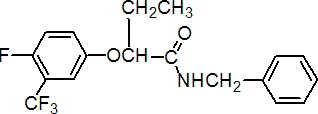
IUPAC name N-benzyl-2-(a,a,a,4-tetrafluoro-m-tolyloxy)butyramide
Chemical Abstracts name 2-[4-fluoro-3-(trifluoromethyl)phenoxy]-N-(phenylmethyl)butanamide
CAS RN [113614-08-7] Development codes UBH-820; UR50601; ASU 95510H (mixture with isoproturon)
PHYSICAL CHEMISTRY
Composition Tech. is >97%. Mol. wt. 355.3 M.f. C18H17F4NO2 Form Fluffy white powder. M.p. 75 °C V.p. 1.1 ´ 10-2 mPa (25 °C) KOW logP = 4.28 Henry 1.1 ´ 10-4 Pa m3 mol-1 S.g./density 1.33 Solubility In water 3.29 mg/l (20 °C). In acetone >600, 1,2-dichloroethane >544, ethyl acetate >571, methanol >473, n-heptane 2.18, xylene 106 (all in g/l, 20 °C). Stability Stable at 130 °C for 5 h. Stable at pH 5, 7 and 9 for 5 d (21 °C). Fairly stable to photolysis.
COMMERCIALISATION
History Reported by S. Takamura et al., Proc. Br. Crop Prot. Conf. - Weeds, 1999, 1, 41. Under development by Ube Industries Ltd; mixture with isoproturon under development by Ube and Stähler Agrochemie GmbH. Patents EP 239414 Manufacturers Ube
APPLICATIONS
Biochemistry Blocks carotenoid biosynthesis, by inhibition of phytoene desaturase. Uses Under development, alone and in mixture with isoproturon, for pre- and early post-emergence control of broad-leaved weeds, such as Veronica persica, Lamium amplexicaule and Viola arvensis, in wheat and barley at 170-255 g/ha. Formulation types SC. Selected products: mixtures: 'Herbaflex' (+ isoproturon) (Stähler, Ube)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin and eyes (rabbits); not a skin sensitiser (guinea pigs). Inhalation LD50 (for rats) >5 ml/l. NOEL (90 d) for rats 29 mg/kg b.w. daily. Other Non-teratogenic. Negative in Ames, gene mutation, cytogenetics and micronucleus tests.
ECOTOXICOLOGY
Data show a low toxicity risk and low bioaccumulation, except for some species of algae. Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Dietary LC50 for bobwhite quail >5200 ppm. Fish LC50 (96 h) for bluegill sunfish 2.69, rainbow trout 1.86 mg/l. Daphnia Acute EC50 (48 h) 1.64 mg/l. Algae EbC50 for Selenastrum 4.45 mg/l. Other aquatic spp. EC50 for Lemna gibba 0.029 mg/l. Bees LD50 (oral and contact) >100 mg/bee. Worms LC50 (14 d) for earthworms 732 mg/kg. Other beneficial spp. Low risk to soil microflora.
ENVIRONMENTAL FATE
Low potential for mobility in soil and rapid degradation minimise potential for leaching into groundwater. Carry-over is not expected after application at approved rates. Animals The main metabolite is the same as in soil. Plants The main metabolite is the same as in soil. Soil/Environment Soil DT50 5.4 d; the main metabolite is the corresponding butanoic acid, formed by cleavage of the amide bond; this metabolite is itself rapidly degraded in soil. Koc 852-1793. Stable to hydrolysis at pH 5-9.
|