|
azafenidin.
Superseded record no. 885
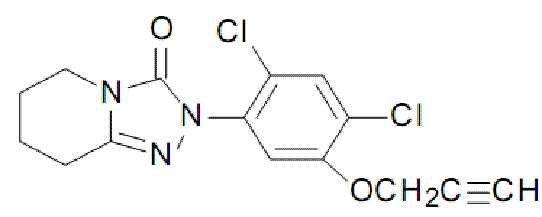
CAS RN [68049-83-2]. Evaluated by DuPont Agricultural Products. Reported by L. Amuti et al., Proc. 1997 Br. Crop Prot. Conf. - Weeds , 1, p. 59.
Common names azafenidin (BSI, pa ISO) IUPAC name 2-(2,4-dichloro-5-prop-2-ynyloxyphenyl)-5,6,7,8-tetrahydro-1,2,4-triazolo[4,3-a]pyridin-3(2H)-one CA name 2-[2,4-dichloro-5-(2-propynyloxy)phenyl]-5,6,7,8-tetrahydro-1,2,4-triazolo[4,3-a ]pyridin-3(2H )-one. Development codes R6447; DPX-R6447; IN-R6447 (all DuPont) M.f. C15H13Cl2N3O2 Composition Tech. is c . 97% pure. Mol. wt. 338.2 Form White powdered solid; (tech. is a rust-coloured solid with a pungent odour). M.p. 168-168.5 °C V.p. 1 ´ 10-6 mPa (20 °C) KOW logP = 2.7 S.g./density 1.4 (20 °C) Solubility In water 16 ppm (pH 7). Stability Stable to hydrolysis; aqueous photolysis DT50 c . 12 h.
Biochemistry Protoporphyrinogen oxidase inhibitor. Mode of action Absorbed through the roots and shoots. Weakly xylem or phloem mobile, hence has limited post-emergence activity. However, it improves the efficacy of other post-emergence herbicides, such as glyphosate, and increases the speed of action of contact herbicides. Uses Intended for use as a pre-emergence, and, in mixture, post-emergence, herbicide in citrus, grapes, olives, sugar cane and other perennial crops; active on both annual and perennial weeds, and applied at 240 g/ha. Formulation types WG. Products 'Evolus' * (Europe) (DuPont); 'Milestone' * (USA) (DuPont) . Details PM12, Entry 43.
|