|
amicarbazone
Herbicide
HRAC C1 WSSA 5; triazolinone
NOMENCLATURE
Common name amicarbazone (BSI, pa ISO)
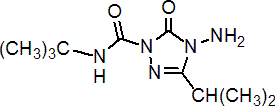
IUPAC name 4-amino-N-tert-butyl-4,5-dihydro-3-isopropyl-5-oxo-1,2,4-1H-triazole-1-carboxamide
Chemical Abstracts name 4-amino-N-(1,1-dimethylethyl)-4,5-dihydro-3-(1-methylethyl)-5-oxo-1H-1,2,4-triazole-1-carboxamide
CAS RN [129909-90-6] Development codes BAY MKH 3586; BAY 314666 (Bayer)
PHYSICAL CHEMISTRY
Mol. wt. 241.3 M.f. C10H19N5O2 Form Colourless crystals. M.p. 137.5 °C V.p. 1.3 ´ 10-3 mPa (20 °C); 3.0 ´ 10-3 mPa (25 °C) KOW logP = 1.18 (pH 4), 1.23 (pH 7), 1.23 (pH 9) (20 °C) Henry 6.8 ´ 10-8 Pa m3 mol-1 (20 °C) S.g./density 1.12 g/ml Solubility In water 4.6 g/l (pH 4-9, 20 °C).
COMMERCIALISATION
History Reported by B. D. Philbrook et al., Proc. Br. Crop Prot. Conf. - Weeds, 1999, 1, 29. Developed by Bayer AG and licensed to Arvesta Corporation. Manufacturers Arvesta
APPLICATIONS
Biochemistry Photosynthesis inhibitor. Mode of action Plant uptake occurs via roots; leaf absorption also occurs on emerged weeds, leading to contact action. This provides burndown as well as residual weed control. Uses Under development for pre-emergence control of annual dicotyledonous weeds, such as Abutilon theophrasti, Chenopodium album, Amaranthus spp., Xanthium strumarium, and Ipomoea spp. in maize at rates up to 500 g/ha. Also for pre- and post- emergence control of annual dicots. and grasses, such as Euphorbia heterophylla, Brachiaria plantaginea and Cenchrus echinatus in sugar cane at rates up to c. 1000 g/ha. Formulation types WG. Selected products: 'Dinamic' (Arvesta)
ANALYSIS
Details of methods for product and residue analysis are available from Arvesta.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for female rats 1015 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin, minimal eye irritation (rabbits); not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats 2.242 mg/l air. Other Not mutagenic, genotoxic, teratogenic, oncogenic.
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Dietary LC50 for bobwhite quail >5000 mg/kg. Fish LC50 (96 h) for bluegill sunfish >129, rainbow trout >120 mg/l. Daphnia LC50 (48 h) >119 mg/l. Other aquatic spp. EC50 for Lemna gibba 226 mg/l. Bees LD50 (oral) 24.8 mg/bee; (contact) >200 mg/bee.
ENVIRONMENTAL FATE
Soil/Environment Hydrolysis DT50 64 d (pH 9, 25 °C); stable at pH 5, 7. Aerobic DT50 in soil 50 d. Preliminary results indicate: soil photolysis DT50 54 d; Koc 23-37; field dissipation DT50 18-24 d; lowest depth detected at >LOQ 12 inch (parent), 24 inch (metabolite). See B. D. Philbrook et al., Proc. Br. Crop Prot. Conf. - Weeds, 1999, 1, 29.
|