|
alpha-cypermethrin
Insecticide
IRAC 3; pyrethroid
NOMENCLATURE
Common name alpha-cypermethrin (BSI, draft E-ISO); alpha-cyperméthrine ((f) draft F-ISO)
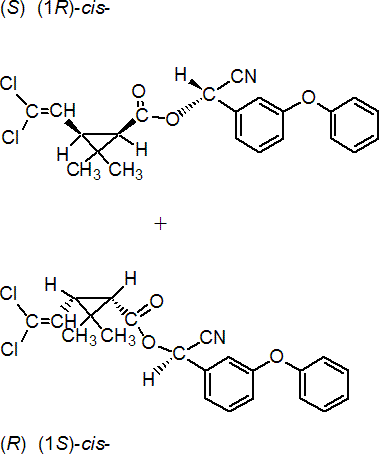
IUPAC name A racemate comprising (S)-a-cyano-3-phenoxybenzyl (1R,3R)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate and (R)-a-cyano-3-phenoxybenzyl (1S,3S)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
Roth: A racemate comprising (S)-a-cyano-3-phenoxybenzyl (1R)-cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate and (R)-a-cyano-3-phenoxybenzyl (1S)-cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
Chemical Abstracts name [1a(S*),3a]-(?-cyano(3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
Other names alfoxylate*; alphamethrin*(rejected common name proposals) CAS RN [67375-30-8] correct stereochemistry; [52315-07-8] (formerly [86752-99-0], [86753-92-6]) cypermethrin (no stereochemistry stated) were sometimes used in Chemical Abstracts Development codes WL 85871 (Shell); FMC 63318; FMC 39391 Official codes OMS 3004
PHYSICAL CHEMISTRY
Composition Tech. grade alpha-cypermethrin is >90% pure m/m, typically >95%. Mol. wt. 416.3 M.f. C22H19Cl2NO3 Form Colourless crystals; (tech. is a white to pale powder, with a weak aromatic odour). M.p. 81.5 ºC (97.3 %) B.p. 200 ºC/9.3 Pa V.p. 2.3 ´ 10-2 mPa (20 ºC) KOW logP = 6.94 (pH 7) Henry 6.9 ´ 10-2 Pa m3 mol-1 (calc.) S.g./density 1.28 (22 ºC) Solubility In water 0.67 (pH 4), 3.97 (pH 7), 4.54 (pH 9), 1.25 (double distilled water) (all in µg/l, 20 °C). In n-hexane 6.5, toluene 596, methanol 21.3, isopropanol 9.6, ethyl acetate 584, acetone:hexane >0.5 (all in g/l, 21 °C); miscible in dichloromethane and in acetone (>10?g/l). Stability Very stable in neutral and acidic media, hydrolysed in strongly alkaline media; DT50 (pH 4, 50 °C) stable over 10 d, (pH 7, 20 °C) 101 d, (pH 9, 20 °C) 7.3 d. Thermally stable up to 220 ºC. Field data indicate that, in practice, it is stable to air. F.p. >80 ºC (closed cup); not highly flammable
COMMERCIALISATION
History Insecticide reported by J. P. Fisher et al. (Proc. Int. Congr. Plant Prot., 10th, 1983, 1, 452). Introduced by Shell International Chemical Co. (now BASF AG) as WL 85871. Manufacturers Agrochem; BASF; Bharat; FMC; Gharda; Meghmani; Mitsu; Rotam; RPG; Sharda; Sundat; Tagros
APPLICATIONS
Biochemistry Acts by preventing transmission of impulses along nerves, brought about by blocking the passage of sodium ions through sodium channels in nerve membranes thus preventing action potentials passing down axons. Typically this intoxication results in a rapid "knockdown" and resultant mortality. Mode of action Non-systemic insecticide with contact and stomach action. Acts on the central and peripheral nervous system in very low doses. Uses Control of a wide range of chewing and sucking insects (particularly Lepidoptera, Coleoptera, and Hemiptera) in fruit (including citrus), vegetables, vines, cereals, maize, beet, oilseed rape, potatoes, cotton, rice, soya beans, forestry, and other crops; applied at 10-15 g/ha. Control of cockroaches, mosquitoes, flies, and other insect pests in public health; and flies in animal houses. Also used as an animal ectoparasiticide. Formulation types EC; SC; TB; UL; WP. Compatibility Compatible with most organophosphorus insecticides. Selected products: 'Fastac' (agronomic use) (BASF); 'Fendona' (public health) (BASF); 'Renegade' (veterinary use) (BASF); 'Alphadhan' (Dhanuka); 'Alphaguard' (Gharda); 'Bestox' (FMC); 'Bestseller' (Agriphar); 'Dominex' (FMC); 'Grander' (Sanonda); 'Hilalpha' (Hindustan); 'Hurricane' (Nagarjuna Agrichem); 'Magic' (public health) (Vapco); 'Merit Alpha' (Crop Health); 'Mig' (Devidayal); 'Stop' (Biostadt); 'Supertak' (Vapco); 'Typic' (Cequisa); 'Vifast' (Vipesco)
OTHER PRODUCTS
'Clameur' (BASF); 'Concord' (BASF); 'Contest' (BASF); 'Mageos' (BASF); 'Vorax' (BASF); 'Agro-Cypethrin Super' (AgroSan); 'Alfamar' (Parry); 'Alfathrin' (Papaeconomou); 'Alfazot' (Azot); 'Alpha-C10' (Standon); 'Alpharin' (Chemvet); 'alpha-Syper' (Agrochem); 'Alphathrin' (Nufarm UK); 'Bala' (FMC); 'Bonsul' (FMC); 'Efitax' (FMC); 'Forward' (RPG); 'Littac' (Sorex); 'Pilar-Alfa' (Pilarquim) mixtures: 'Tenopa' (+ flufenoxuron) (BASF, Sorex); 'Maxihit' (+ chlorpyrifos) (Crop Health); 'Multi-Fog ATP' (+ piperonyl butoxide+ tetramethrin) (Trithin) Discontinued products: 'Acquit' * (DuPont) mixtures: 'Supercord' * (+ piperonyl butoxide) (Shell)
ANALYSIS
Product analysis by glc with FID (CIPAC Handbook, 1998, H, 14). Residues determined by glc with ECD. (Validation determined with capillary gc with MSD). Soil, and body fluids and tissues determined by capillary gc with ECD; water determined by gc with ECD; air determined by capillary gc with NPD. Details available from BASF. Analysis of pyrethroids reviewed by E. Papadopoulou-Mourkidou in Comp. Analyt. Profiles.
MAMMALIAN TOXICOLOGY
Reviews JECFA 47 (see part 2 of the Bibliography). See A. J. Gray & D. M. Soderlund, Chapt. 5 in "Insecticides". Oral Acute oral LD50 for rats 57 mg/kg (in corn oil). Skin and eye Acute percutaneous LD50 for rats and rabbits >2000 mg tech./kg; minimal irritant to the eyes of rabbits. Inhalation LC50 (4 h, nose only) for rats >0.593 mg/l (maximum attainable concentration). NOEL NOAEL (1 y) for dogs >60 mg/kg diet (1.5 mg/kg b.w. daily). ADI 0.02 mg/kg b.w. (JECFA evaluation) [1996]; 0.015 mg/kg b.w. (BASF). Other Non-mutagenic. Toxic to CNS and peripheral motor nerves; neuro-behavioural changes reversible within 3 days following a single dose. Acute rat study NOAEL 4 mg/kg b.w. (in corn oil); 4 week oral rat study NOAEL 10 mg/kg b.w. daily (in DMSO). May cause paresthesia. Toxicity class WHO (a.i.) II; EPA (formulation) II
ECOTOXICOLOGY
Birds LD50 for Northern bobwhite >2025 mg/kg; LC50 for Northern bobwhite >5000 mg/kg food. Reproductive toxicity NOEC (20 weeks) forNorthern bobwhite 150 mg/kg of food. Fish LC50 (96 h) for rainbow trout 2.8 mg/l; due to rapid loss from water, no toxic effect to fish is observed under field conditions. NOEC from early life stage test (34 d) 0.03 mg/l. Daphnia EC50 (48 h) 0.1-0.3 mg/l. Algae EC50 (96 h) >100 mg/l. Other aquatic spp. NOEC (28 d) for Chironomus riparius larvae 0.024 mg/l. Mesocosm study (1999): EAC 0.015 mg/l. Bees Toxic to bees. LD50 (24 h) 0.059 mg/bee; LC50 (24 h) 0.033 mg/bee. No toxic effect under field conditions. Worms LD50 (14 d) for earthworms >100 mg/kg artificial soil. No effect on earthworm reproduction was observed at a treatment representing 300 g/ha. Other beneficial spp. For Typhlodromus pyri at 15 g/ha >85% mortality; for Pardosa sp. at 0.21 and 0.6 g/ha <30% mortality, at 1.5 g/ha 60% mortality, at 30 g/ha 100% mortality; for Poecilus cupreus at 1.2 and 30 g/ha 0% and 17% mortality; for Aleochara bilineata at 1.2 g/ha 21% mortality, harmless at £0.7 g/ha; LR50 for Orius 0.1 g/ha, for Aphidius rhopalosiphi 0.9 g/ha.
ENVIRONMENTAL FATE
EHC 142 (WHO, 1992) EHC 142 notes that, although highly toxic to fish, this is not realised under field conditions where rapid loss from water allows recovery of affected populations. Animals See cypermethrin. Clearance time 7.8 d. Level of residues in organisms after the 28 day depuration phase 8% of the maximum level. Soil/Environment Undergoes degradation in soil, DT50 c. 13 w in loamy soil. See also cypermethrin (q.v.).
|