|
acrinathrin
Acaricide, insecticide
IRAC 3; pyrethroid
NOMENCLATURE
Common name acrinathrin (BSI, draft E-ISO); acrinathrine (draft F-ISO)
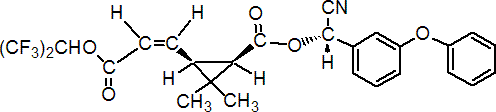
IUPAC name (S)-a-cyano-3-phenoxybenzyl (Z)-(1R,3S)-2,2-dimethyl-3-[2-(2,2,2-trifluoro-1-trifluoromethylethoxycarbonyl)vinyl]cyclopropanecarboxylate
Roth: (S)-a-cyano-3-phenoxybenzyl (Z)-(1R-cis)-2,2-dimethyl-3-[2-(2,2,2-trifluoro-1-trifluoromethylethoxycarbonyl)vinyl]cyclopropanecarboxylate
Chemical Abstracts name cyano(3-phenoxyphenyl)methyl 2,2-dimethyl-3-[3-oxo-3-[2,2,2-trifluoro-1-(trifluoromethyl)ethoxy]-1-propenyl]cyclopropanecarboxylate
CAS RN [101007-06-1] as defined; [103833-18-7] unstated stereochemistry Development codes RU 38 702 (Roussel Uclaf); HOE 076003 (Hoechst); AE F 076003 (AgrEvo); NU 702
PHYSICAL CHEMISTRY
Composition Material is a single isomer. Mol. wt. 541.4 M.f. C26H21F6NO5 Form White powder (tech.). M.p. 81.5 ºC (pure); 82 ºC (tech.) V.p. 4.4 ´ 10-5 mPa (20 °C) KOW logP = 5.6 (a.i., 25 ºC) Henry 4.8 ´ 10-2 Pa m3 mol-1 (calc.) Solubility In water £0.02 mg a.i./l (25 ºC). In acetone, chloroform, dichloromethane, ethyl acetate, dimethylformamide >500, di-isopropyl ether 170, ethanol 40, hexane 10, n-octanol 10 (all in g a.i./l). Stability Stable in acid, but hydrolysis and epimerisation more important at pH >7. DT50 >1 y (pH 5, 50 ºC), 30 d (pH 7, 30 ºC) 15 d (pH 9, 20 ºC), 1.6 d (pH 9, 37 ºC). Stable for 7 d under 100 W light (tech.). Specific rotation [a]D20 +17.5?
COMMERCIALISATION
History Acaricide and insecticide reported by J. R. Tessier et al. (IUPAC Pestic. Chem., 1983, 5, 95). Introduced in France (1990) by Roussel Uclaf (now Bayer CropScience). Licensed to Cheminova in 2003. Patents EP 48186; FR 2486073 Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Acts on the nervous system of insects, disturbs the function of neurons by interaction with the sodium channel. Mode of action Contact and stomach action. Uses An ingested and contact acaricide effective against a wide range of phytophagous mites on citrus, cotton, fruit, hops, ornamentals, soya beans, tobacco, vegetables and vines. It also shows insecticidal properties, in particular with high efficacy on thrips species on fruit trees, vines and vegetables. Formulation types EC; EW; SC; WP. Compatibility May not be compatible with alkaline products. Selected products: 'Rufast' (Cheminova, Bayer CropScience)
OTHER PRODUCTS
'Ardent' (Cheminova, Bayer CropScience); 'Orytis' (Cheminova, Bayer CropScience); 'Taflis' (Cheminova, Bayer CropScience); 'Tagloo' (Cheminova, Bayer CropScience)
ANALYSIS
Product analysis by hplc. Residues in plants, soil and water determined by glc with ECD.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats and mice >5000 mg/kg (for tech. in corn oil). Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to eyes and skin of rabbits. Not skin-sensitising to guinea pigs. Inhalation LC50 (4 h) for rats 1.6 mg/l air. NOEL (90 d) for male rats 2.4 mg/kg b.w., for female rats 3.1 mg/kg b.w.; (1 y) for dogs 3 mg/kg b.w. Non-mutagenic and non-teratogenic in rats (2 mg/kg b.w. daily) and rabbits (15 mg/kg b.w. daily). ADI 0.02 mg/kg. Other Low solubility in water and high adsorption on soil mean that low LC50 or LD50 values under laboratory conditions do not present significant hazard under practical field conditions. Toxicity class WHO (a.i.) U; EPA (formulation) IV
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2250, mallard ducks >1000 mg/kg. LC50 (8 d) for bobwhite quail 3275, mallard ducks 4175 mg/kg diet. Fish LC50 for rainbow trout 5.66, mirror carp 0.12 mg/l. Daphnia LC50 (48 h) 0.57 mg/l. Algae EC50 (96 h) for green algae >0.82 mg/l. Bees LC50 (48 h) (oral) 150-200 ng/bee; (48 h) (contact) 200-500 ng/bee. Worms LC50 (14 d) for earthworms >1000 mg/kg; NOEC biomass 1.6 mg/kg.
ENVIRONMENTAL FATE
Animals No metabolites found representing >10% of parent compound. Plants The main residue is the parent compound. Soil/Environment Strongly adsorbed onto soil and immobile (irrespective of pH and o.m. content); Kd 2460-2780; Koc 127 500-319 610. Soil column leaching: <1% of applied acrinathrin found in leachate. DT50 5-100 d (4 soil types). DT50 under aerobic conditions (pH 6.2, o.m. 3.1%) 52 d.
|