|
aclonifen
Herbicide
HRAC F3 WSSA 13; diphenyl ether (cbi)
NOMENCLATURE
Common name aclonifen (BSI, draft E-ISO); aclonifène ((m) draft F-ISO)
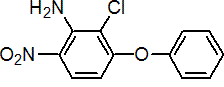
IUPAC name 2-chloro-6-nitro-3-phenoxyaniline
Chemical Abstracts name 2-chloro-6-nitro-3-phenoxybenzenamine
CAS RN [74070-46-5] EEC no. 277-704-1 Development codes CME 127 (Celamerck); KUB 3359; LE84493 (Rhône-Poulenc)
PHYSICAL CHEMISTRY
Composition ³95% pure. Mol. wt. 264.7 M.f. C12H9ClN2O3 Form Yellow crystals. M.p. 81-82 ºC V.p. 1.6 ´ 10-2 mPa (20 ºC) KOW logP = 4.37 Henry 3.2 ´ 10-3 Pa m3 mol-1 (20 °C) S.g./density 1.46 g/cm3 Solubility In water 1.4 mg/l (20 ºC). In methanol 50, hexane 4.5, toluene 390 (all in g/kg, 20 ºC). Stability Slowly decomposes when exposed to light.
COMMERCIALISATION
History Herbicide reported by W. Buck et al. (Proc. Int. Congr., Plant Prot., 10th, 1983, 1, 307). Introduced by Celamerck GmbH & Co. (became Shell Agrar) and later sold to Rhône-Poulenc Agrochimie (now Bayer CropScience). Patents US 4394159; DE 2831262 (to Celamerck) Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Inhibits carotenoid biosynthesis; target enzyme not known. Mode of action Systemic, selective herbicide. Uses Pre-emergence control of grass and broad-leaved weeds in winter wheat, potatoes, sunflowers, peas, carrots, maize, and other crops. Phytotoxicity Non-phytotoxic to potatoes, sunflowers, and peas. May be phytotoxic to cereals and maize at high dose rates. Formulation types SC. Selected products: 'Challenge' (Bayer CropScience)
OTHER PRODUCTS
'Bandur' (Bayer CropScience); 'Fenix' (Bayer CropScience) mixtures: 'Acajou' (+ isoxaflutole) (Bayer CropScience); 'Lagon' (+ isoxaflutole) (Bayer CropScience); 'Manager' (+ alachlor) (Bayer CropScience); 'Nikeyl' (+ flurtamone) (Bayer CropScience) Discontinued products: 'Bandren' * (Rhône-Poulenc)
ANALYSIS
Product and residue analysis by glc. Details from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats and mice >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >5000 mg/kg. Slight skin irritant; non-irritating to eyes (rabbits). Inhalation LC50 (4 h) for rats >5.06 mg/l air. NOEL (90 d) for rats 28 mg/kg b.w. daily; (180 d) for dogs 12.5 mg/kg b.w. daily. ADI 0.02 mg/kg (France). Other Non-mutagenic in the Ames test. Not embryotoxic or teratogenic in rats. Does not affect reproduction in rats at 2000 ppm over 2 generations. Toxicity class WHO (a.i.) U EC classification N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 for Japanese quail and canaries >15 000 mg/kg b.w. Fish LC50 (96 h) for rainbow trout 0.67, carp 1.7 mg/l. Daphnia EC50 (48 h) 2.5 mg/l. Algae EC50 (96 h) 6.9 mg/l. Bees Not toxic to bees. LD50 (oral) >100 mg/bee. Worms LC50 (14 d) 300 mg/kg.
ENVIRONMENTAL FATE
Animals In rats, following oral administration, 62-65% is excreted in the urine, principally in the form of polar compounds. No bioaccumulation. Plants In plants, hydroxylation occurs on both benzene rings. DT50 c. 14 d. Soil/Environment In sterile water, stable between pH 3 to 9. DT50 in water in presence of micro-organisms c. 1 mo. In soil, DT50 36-80 d (22 ºC). Koc 5318-12 164. Unlikely to leach: -0.13 < GUS < 0.64.
|