|
acibenzolar-S-methyl
Plant activator
FRAC P1, P1; benzothiadiazole
NOMENCLATURE
Common name acibenzolar-S-methyl (BSI, pa ISO)
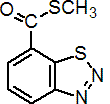
IUPAC name S-methyl benzo[1,2,3]thiadiazole-7-carbothioate
Chemical Abstracts name 1,2,3-benzothiadiazole-7-carbothioic acid S-methyl ester
CAS RN [135158-54-2]; [126448-41-7] (acid) Development codes CGA 245704 (Ciba-Geigy)
PHYSICAL CHEMISTRY
Composition Tech. is 98.6% pure. Mol. wt. 210.3 M.f. C8H6N2OS2 Form White to beige fine powder with a burnt-like odour. M.p. 132.9 °C B.p. c. 267 °C V.p. 4.6 ´ 10-1 mPa (25 °C) KOW logP = 3.1 (25 °C) Henry 1.3 ´ 10-2 Pa m3 mol-1 (calc.) S.g./density 1.54 (22 °C) Solubility In water 7.7 mg/l (pH 7.5-7.9, 25 °C). In methanol 4.2, ethyl acetate 25, n-hexane 1.3, toluene 36, n-octanol 5.4, acetone 28, dichloromethane 160 (all in g/l, 25 °C). Stability Hydrolysis DT50 (20 °C) 3.8 y (pH 5), 23 w (pH 7), 19.4 h (pH 9).
COMMERCIALISATION
History Reported by W. Ruess et al., XIII Int. Plant Prot. Congr., The Hague, Netherlands, July 2-7, 1995; idem, Proc. Br. Crop Prot. Conf. - Pests Dis., 1996, 1, 53. Introduced by Ciba-Geigy AG (now Syngenta AG) in Germany and Switzerland in 1996. Patents EP 313512 Manufacturers Syngenta
APPLICATIONS
Biochemistry Acts as a functional analogue of the natural signal molecule for systemic activated resistance, salicylic acid (H. Kessmann et al.,Proc. Br. Crop Prot. Conf. - Pests Dis., 1996, 3, 961). Mode of action Activates the host plant's natural defence mechanism ("systemic activated resistance" (SAR)). Has no intrinsic fungicidal activity. Uses For use against a range of fungal infections of wheat. Must be applied as a protective treatment or early in the disease progress. Under development against a range of diseases in rice, bananas, vegetables and tobacco. Formulation types SC; WG. Selected products: 'Bion' (Syngenta)
OTHER PRODUCTS
'Actigard' (Syngenta); 'Blockade' (Syngenta); 'Boost' (Syngenta) mixtures: 'Bion MX' (+ metalaxyl-M) (Syngenta)
ANALYSIS
Product analysis by glc with FID. Residues determined by hplc. Details available from Syngenta.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Not irritating to skin or eyes (rabbits). Skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >5000 mg/m3 air. NOEL (2 y) for rats 8.5 mg/kg b.w. daily; (1.5 y) for mice 11 mg/kg b.w. daily; (1 y) for dogs 5 mg/kg b.w. daily. ADI 0.05 mg/kg b.w. Other Not oncogenic, not mutagenic; of no teratogenic relevance for humans. Toxicity class WHO (a.i.) U (company classification); EPA (formulation) III EC classification Xi; R36/37/38| R43| N; R50, R53
ECOTOXICOLOGY
Birds LD50 (14 d) for mallard ducks and bobwhite quail >2000 mg/kg. LC50 (8 d) for mallard ducks and bobwhite quail >5200 mg/kg. Fish LC50 (96 h) for rainbow trout 0.4, bluegill sunfish 2.8, sheepshead minnow 1.7 mg/l. Daphnia LC50 (48 h) 2.4 mg/l. Algae EbC50 (72 h) for Scenedesmus subspicatus 1.7 mg/l. Other aquatic spp. LC50 (96 h) for mysid shrimp 0.88 mg/l. Bees LD50 (48 h) (oral) 128.3 mg/bee; (contact) 100 mg/bee. Worms LC50 (14 d) for Eisenia foetida >1000 mg/kg soil. Other beneficial spp. Harmless to predator bug and mite, ground beetle and parasitic wasp (IOBC). No effect on soil respiration at 300 g/ha.
ENVIRONMENTAL FATE
Animals After oral administration, acibenzolar-S-methyl is rapidly absorbed and also rapidly almost completely eliminated with urine and faeces. The metabolic pathways are independent of sex, pre-treatment or dose level administered. Residues in tissues were generally low and there was no evidence of accumulation or retention of acibenzolar-S-methyl or its metabolites. Plants The metabolism proceeds via hydrolysis with subsequent conjugation with sugars, or by oxidation of the phenyl ring followed by sugar conjugation. Soil/Environment In soil, the compound dissipates via hydrolysis; DT50 0.3 d (pH 9). The product further degrades, DT50 20 d; metabolites become completely degraded and mineralised (pH 9). Strong adsorption to soil, low mobility, Koc 1394 ml/g. In water, for acibenzolar-S-methyl, DT50 <1 d; for the hydrolysis product, DT50 8 d.
|