|
quizalofop-P-tefuryl
Herbicide
HRAC A WSSA 1; aryloxyphenoxypropionate
NOMENCLATURE
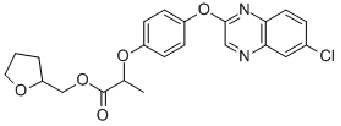
quizalofop-P-tefuryl
Common name quizalofop-P-tefuryl
IUPAC name (?-tetrahydrofurfuryl-(R)-2-[4-(6-chloroquinoxalin-2-yloxy)phenoxy]propionate
Chemical Abstracts name (RS)-2-tetrahydrofuranylmethyl (R)-2-[4-(6-chloro-2-quinoxalinyloxy)phenoxy]propanoate
CAS RN [119738-06-6] Development codes UBI C4874 (Uniroyal)
PHYSICAL CHEMISTRY
quizalofop-P-tefuryl
Mol. wt. 428.9 M.f. C22H21ClN2O5 Form Thick yellow liquid, which can crystallise upon standing at room temperature. M.p. 59-68 ºC; (tech. is a liquid) V.p. 7.9 ´ 10-3 mPa (25 ºC, gas saturation method) KOW logP = 4.32 (25 ºC) Henry 9.02 ´ 10-4 Pa m3 mol-1 (calc.) S.g./density 1283 kg/m3 (21.5 ºC) Solubility In water 4 mg/l (25 ºC). In toluene 652, hexane 12, methanol 64 (all in g/l). Stability Stable for ³14 d at 55 ºC; stable in package for ³1 y at 25 ºC. Stable ³7 d in sunlight. In water, DT50 (22 ºC) 8.1 d (pH 5), 82 d (pH 6), 431 min (pH 9). Specific rotation [a]D 31.9? tech. 28-32? pKa 1.25 (25 ºC) F.p. 132 °C (Setaflash) Other properties Surface tension 69.3 mN/m (21 °C)
COMMERCIALISATION
History The herbicidal enantiomer ester quizalofop-P-ethyl introduced by Nissan Chemical Industries, Ltd and quizalofop-P-tefuryl ((?-tetrahydrofurfuryl ester) (A. R. Bell & A. S. Peddie, Proc. 1989 Br. Crop Prot. Conf. - Weeds, 1, 65) by Uniroyal Chemical Co., Inc. (now part of Crompton Corp.).
quizalofop-P-tefuryl
Manufacturers Crompton; IPESA
APPLICATIONS
Biochemistry Fatty acid synthesis inhibitor (inhibition of acetyl CoA carboxylase) Mode of action Systemic herbicide, absorbed from the leaf surface, with translocation throughout the plant, moving in both the xylem and phloem, and accumulating in the meristematic tissue.
quizalofop-P-tefuryl
Uses Control of annual grasses such as Avena fatua and Alopecurus myosuroides, and perennial grasses such as Sorghum halepense and Elymus repens, in potatoes, flax, oilseed rape, peas, sugar beet, cotton, and soya beans. Applied at 30-240 g/ha. Formulation types EC. Compatibility Certain post-emergence broad-leaved herbicides are incompatible with quizalofop-P-tefuryl. Selected products: 'Panarex' (Crompton); 'Pantera' (Crompton); 'Rango' (Crompton); 'Sotus' (Crompton); 'Sector-T' (IPESA)
OTHER PRODUCTS
quizalofop-P-tefuryl
Discontinued products: 'Logico' * (IPESA)
ANALYSIS
quizalofop-P-tefuryl
Details available from Crompton.
MAMMALIAN TOXICOLOGY
quizalofop-P-tefuryl
Oral Acute oral LD50 for rats 1012 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Mild eye irritation; non-irritating to skin (rabbits). Non-sensitising to skin (guinea pigs). NOEL In chronic oncogenic feeding studies, NOEL (2 y) for rats 1.3, dogs (1 y) 25-30 mg/kg b.w. daily. ADI 0.01 mg/kg. Toxicity class WHO (a.i.) II; EPA (formulation) III EC classification R68| R61| R62| Xn; R22, R48/22| N; R50, R53
ECOTOXICOLOGY
quizalofop-P-tefuryl
Birds Acute oral LD50 for bobwhite quail and mallard ducks >2150 mg/kg. LC50 (8 d) for bobwhite quail and mallard ducks >5000 ppm. Fish LC50 (96 h) for trout 0.51, sunfish 0.23 mg/l. Daphnia LC50 (48 h) >1.5 mg/l. Algae LC50 (5 d) for Selenastrum >1.9 mg/l. Other aquatic spp. EC50 (72 h) for Navicula pelliculosa 1.3 mg/l. Bees LD50 >100 mg/bee. Worms LC50 (14 d) >1000 mg/kg.
ENVIRONMENTAL FATE
quizalofop-P-tefuryl
Animals Extensively degraded by hydrolysis, hydroxylation and conjugation. Plants Rapidly hydrolysed to quizalofop. Soil/Environment DT50 1.9-22.0 h.
|
